当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of a UGT84 Family Glycosyltransferase Provides New Insights into Substrate Binding and Reactivity of Galloylglucose Ester-Forming UGTs
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acs.biochem.7b00946 Alexander E. Wilson 1 , Xiaoxue Feng 1 , Nadia N. Ono 1 , Doron Holland 2 , Rachel Amir 3 , Li Tian 1, 4, 5
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acs.biochem.7b00946 Alexander E. Wilson 1 , Xiaoxue Feng 1 , Nadia N. Ono 1 , Doron Holland 2 , Rachel Amir 3 , Li Tian 1, 4, 5
Affiliation
|
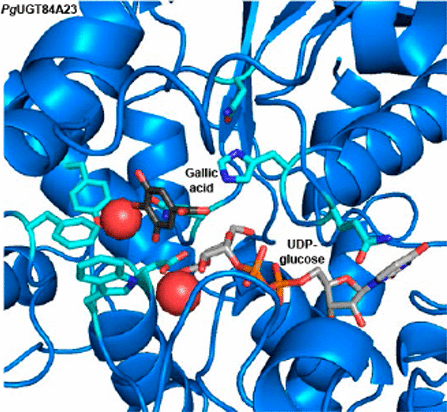
Galloylated plant specialized metabolites play important roles in plant–environment interactions and in the promotion of human and animal health. The galloylation reactions are mediated by the formation of galloylglucose esters from gallic acid and UDP-glucose, catalyzed by the plant UGT84 family glycosyltransferases. To explore and exploit the structural determinants of UGT84 activities, we performed homology modeling and substrate docking of PgUGT84A23, a galloylglucose ester-forming family 84 UGT, as well as sequence comparisons of PgUGT84A23 with other functionally characterized plant UGTs. By employing site-directed mutagenesis of candidate amino acids, enzyme assays with analogous substrates, and kinetic analysis, we elucidated key amino acid sites for PgUGT84A23 substrate binding and reactivity. The galloylglucose ester-forming UGT84s have not been shown to glycosylate genistein (an isoflavonoid) in vivo. Unexpectedly, amino acids highly conserved among UGT84s that affect specifically the binding of genistein but not gallic acid or other tested sugar acceptors were identified. This result suggests that genistein may resemble the substrate profile for the enzyme ancestor of the galloylglucose ester-forming UGTs and recruited during transition from a general to a more specialized defense function. Overall, a better understanding of the structure–function relationship of UGT84s will facilitate enzyme engineering for the production of pharmaceutically and industrially valuable glycosylated compounds.
中文翻译:

UGT84家族糖基转移酶的表征为形成没食子酰基葡萄糖酯的UGT提供底物结合和反应性的新见解。
镀锌植物专用代谢物在植物与环境之间的相互作用以及促进人类和动物健康方面发挥着重要作用。没食子酸酯化反应是由植物UGT84家族糖基转移酶催化由没食子酸和UDP-葡萄糖形成没食子酰基葡萄糖酯介导的。为了探索和利用UGT84活性的结构决定因素,我们进行了同源建模和Pg UGT84A23,一个形成甲酰基葡萄糖酯的家族84 UGT的底物对接,以及Pg UGT84A23与其他功能表征的植物UGT的序列比较。通过采用候选氨基酸的定点诱变,具有相似底物的酶分析和动力学分析,我们阐明了Pg的关键氨基酸位点UGT84A23底物结合和反应性。尚未显示形成没食子酰基葡萄糖酯的UGT84s在体内将染料木黄酮(异黄酮)糖基化。出乎意料的是,鉴定出了UGT84s中高度保守的氨基酸,这些氨基酸特别影响染料木黄酮的结合,而不影响没食子酸或其他经过测试的糖受体。该结果表明,染料木黄酮可能类似于形成没食子酰基葡萄糖酯的UGT的酶祖先的底物概况,并且在从一般防御功能过渡到更专门的防御功能的过程中被吸收。总体而言,对UGT84s的结构与功能关系的更好理解将有助于酶工程技术生产药学和工业上有价值的糖基化化合物。
更新日期:2017-11-22
中文翻译:

UGT84家族糖基转移酶的表征为形成没食子酰基葡萄糖酯的UGT提供底物结合和反应性的新见解。
镀锌植物专用代谢物在植物与环境之间的相互作用以及促进人类和动物健康方面发挥着重要作用。没食子酸酯化反应是由植物UGT84家族糖基转移酶催化由没食子酸和UDP-葡萄糖形成没食子酰基葡萄糖酯介导的。为了探索和利用UGT84活性的结构决定因素,我们进行了同源建模和Pg UGT84A23,一个形成甲酰基葡萄糖酯的家族84 UGT的底物对接,以及Pg UGT84A23与其他功能表征的植物UGT的序列比较。通过采用候选氨基酸的定点诱变,具有相似底物的酶分析和动力学分析,我们阐明了Pg的关键氨基酸位点UGT84A23底物结合和反应性。尚未显示形成没食子酰基葡萄糖酯的UGT84s在体内将染料木黄酮(异黄酮)糖基化。出乎意料的是,鉴定出了UGT84s中高度保守的氨基酸,这些氨基酸特别影响染料木黄酮的结合,而不影响没食子酸或其他经过测试的糖受体。该结果表明,染料木黄酮可能类似于形成没食子酰基葡萄糖酯的UGT的酶祖先的底物概况,并且在从一般防御功能过渡到更专门的防御功能的过程中被吸收。总体而言,对UGT84s的结构与功能关系的更好理解将有助于酶工程技术生产药学和工业上有价值的糖基化化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号