当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deciphering Conformational Changes Associated with the Maturation of Thrombin Anion Binding Exosite I
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acs.biochem.7b00970 Ramya Billur 1 , David Ban 2 , T. Michael Sabo 2 , Muriel C. Maurer 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acs.biochem.7b00970 Ramya Billur 1 , David Ban 2 , T. Michael Sabo 2 , Muriel C. Maurer 1
Affiliation
|
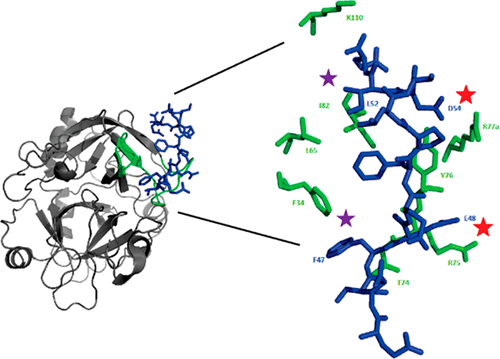
Thrombin participates in procoagulation, anticoagulation, and platelet activation. This enzyme contains anion binding exosites, ABE I and ABE II, which attract regulatory biomolecules. As prothrombin is activated to thrombin, pro-ABE I is converted into mature ABE I. Unexpectedly, certain ligands can bind to pro-ABE I specifically. Moreover, knowledge of changes in conformation and affinity that occur at the individual residue level as pro-ABE I is converted to ABE I is lacking. Such changes are transient and were not captured by crystallography. Therefore, we employed nuclear magnetic resonance (NMR) titrations to monitor development of ABE I using peptides based on protease-activated receptor 3 (PAR3). Proton line broadening NMR revealed that PAR3 (44–56) and more weakly binding PAR3G (44–56) could already interact with pro-ABE I on prothrombin. 1H–15N heteronuclear single-quantum coherence NMR titrations were then used to probe binding of individual 15N-labeled PAR3G residues (F47, E48, L52, and D54). PAR3G E48 and D54 could interact electrostatically with prothrombin and tightened upon thrombin maturation. The higher affinity for PAR3G D54 suggests the region surrounding thrombin R77a is better oriented to bind D54 than the interaction between PAR3G E48 and thrombin R75. Aromatic PAR3G F47 and aliphatic L52 both reported on significant changes in the chemical environment upon conversion of prothrombin to thrombin. The ABE I region surrounding the 30s loop was more affected than the hydrophobic pocket (F34, L65, and I82). Our NMR titrations demonstrate that PAR3 residues document structural rearrangements occurring during exosite maturation that are missed by reported X-ray crystal structures.
中文翻译:

与凝血酶阴离子结合异位酶I的成熟有关的构象变化的破译
凝血酶参与促凝,抗凝和血小板活化。该酶含有吸引结合性生物分子的结合阴离子的外来分子ABE I和ABE II。当凝血酶原被活化为凝血酶时,pro-ABE I被转化为成熟的ABEI。出乎意料的是,某些配体可以特异性结合pro-ABEI。而且,缺乏关于将原ABE I转化为ABE I时在各个残基水平上发生的构象和亲和力变化的知识。这样的变化是暂时的,没有被晶体学捕获。因此,我们使用核磁共振(NMR)滴定法来监测ABE I的开发,并使用基于蛋白酶激活受体3(PAR3)的肽。质子谱变宽的NMR显示PAR3(44-56)和较弱结合的PAR3G(44-56)可能已经与凝血酶原上的pro-ABE I相互作用。然后使用1 H– 15 N异核单量子相干NMR滴定法来探测单个15N标记的PAR3G残基(F47,E48,L52和D54)。PAR3G E48和D54可以与凝血酶原发生静电相互作用,并在凝血酶成熟时变紧。对PAR3G D54的更高亲和力表明,与PAR3G E48和凝血酶R75之间的相互作用相比,凝血酶R77a周围的区域更易于结合D54。芳香族PAR3G F47和脂肪族L52均报告了凝血酶原转化为凝血酶后化学环境的显着变化。30s环周围的ABE I区比疏水性囊袋(F34,L65和I82)受到的影响更大。我们的NMR滴定表明,PAR3残基记录了异位成熟过程中发生的结构重排,但被报道的X射线晶体结构遗漏了。
更新日期:2017-11-22
中文翻译:

与凝血酶阴离子结合异位酶I的成熟有关的构象变化的破译
凝血酶参与促凝,抗凝和血小板活化。该酶含有吸引结合性生物分子的结合阴离子的外来分子ABE I和ABE II。当凝血酶原被活化为凝血酶时,pro-ABE I被转化为成熟的ABEI。出乎意料的是,某些配体可以特异性结合pro-ABEI。而且,缺乏关于将原ABE I转化为ABE I时在各个残基水平上发生的构象和亲和力变化的知识。这样的变化是暂时的,没有被晶体学捕获。因此,我们使用核磁共振(NMR)滴定法来监测ABE I的开发,并使用基于蛋白酶激活受体3(PAR3)的肽。质子谱变宽的NMR显示PAR3(44-56)和较弱结合的PAR3G(44-56)可能已经与凝血酶原上的pro-ABE I相互作用。然后使用1 H– 15 N异核单量子相干NMR滴定法来探测单个15N标记的PAR3G残基(F47,E48,L52和D54)。PAR3G E48和D54可以与凝血酶原发生静电相互作用,并在凝血酶成熟时变紧。对PAR3G D54的更高亲和力表明,与PAR3G E48和凝血酶R75之间的相互作用相比,凝血酶R77a周围的区域更易于结合D54。芳香族PAR3G F47和脂肪族L52均报告了凝血酶原转化为凝血酶后化学环境的显着变化。30s环周围的ABE I区比疏水性囊袋(F34,L65和I82)受到的影响更大。我们的NMR滴定表明,PAR3残基记录了异位成熟过程中发生的结构重排,但被报道的X射线晶体结构遗漏了。









































 京公网安备 11010802027423号
京公网安备 11010802027423号