当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetic Isotope Effects and Transition State Structure for Hypoxanthine-Guanine-Xanthine Phosphoribosyltransferase from Plasmodium falciparum
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acs.biochem.7b01027 Rodrigo G. Ducati 1 , Ross S. Firestone 1 , Vern L. Schramm 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acs.biochem.7b01027 Rodrigo G. Ducati 1 , Ross S. Firestone 1 , Vern L. Schramm 1
Affiliation
|
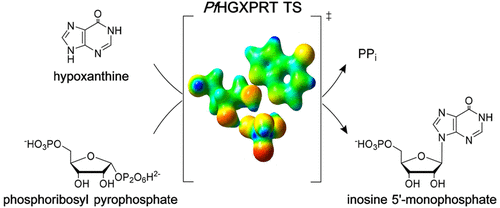
Plasmodium falciparum parasites are purine auxotrophs that rely exclusively on the salvage of preformed purines from their human hosts to supply the requirement for purine nucleotides. Hypoxanthine-guanine-xanthine phosphoribosyltransferase (HGXPRT) catalyzes the freely reversible Mg2+-dependent conversion of 6-oxopurine bases to their respective nucleotides and inorganic pyrophosphate. The phosphoribosyl group is derived from 5-phospho-α-d-ribosyl 1-pyrophosphate (PRPP). The enzyme from malaria parasites (PfHGXPRT) is essential as hypoxanthine is the major precursor in purine metabolism. We used specific heavy atom labels in PRPP and hypoxanthine to measure primary (1-14C and 9-15N) and secondary (1-3H and 7-15N) intrinsic kinetic isotope effect (KIE) values for PfHGXPRT. Intrinsic isotope effects contain information for understanding enzymatic transition state properties. The transition state of PfHGXPRT was explored by matching KIE values predicted from quantum mechanical calculations to the intrinsic values determined experimentally. This approach provides information about PfHGXPRT transition state bond lengths, geometry, and atomic charge distribution. The transition state structure of PfHGXPRT was determined in the physiological direction of addition of ribose 5-phosphate to hypoxanthine by overcoming the chemical instability of PRPP. The transition state for PfHGXPRT forms nucleotides through a well-developed and near-symmetrical DN*AN, SN1-like transition state.
中文翻译:

恶性疟原虫次黄嘌呤-鸟嘌呤-黄嘌呤磷酸核糖基转移酶的动力学同位素效应和过渡态结构
恶性疟原虫寄生虫是嘌呤营养缺陷型,仅依赖于人类宿主中预制嘌呤的抢救来提供嘌呤核苷酸的需求。次黄嘌呤-鸟嘌呤-黄嘌呤磷酸核糖基转移酶(HGXPRT)催化6-氧嘌呤碱基向其各自的核苷酸和无机焦磷酸盐的自由可逆Mg 2+依赖性转化。磷酸核糖基衍生自5-磷酸-α- d-核糖基1-焦磷酸酯(PRPP)。由于次黄嘌呤是嘌呤新陈代谢的主要前体,因此来自疟疾寄生虫的酶(Pf HGXPRT)是必不可少的。我们使用了特定的重原子标记在PRPP和次黄嘌呤测定初级(1- 14 C和9- 15N)和次级(1- 3 H和7- 15 N)为固有动力学同位素效应(KIE)值Pf的HGXPRT。内在同位素效应包含用于理解酶促过渡态性质的信息。通过将量子力学计算中预测的KIE值与实验确定的内在值相匹配,探索了Pf HGXPRT的过渡态。此方法提供有关Pf HGXPRT过渡态键长,几何形状和原子电荷分布的信息。Pf的过渡态结构通过克服PRPP的化学不稳定性,在向次黄嘌呤中添加5磷酸核糖的生理方向确定了HGXPRT。为过渡状态Pf的HGXPRT通过发达和近对称d形成核苷酸Ñ * A Ñ,S Ñ 1状过渡状态。
更新日期:2017-11-22
中文翻译:

恶性疟原虫次黄嘌呤-鸟嘌呤-黄嘌呤磷酸核糖基转移酶的动力学同位素效应和过渡态结构
恶性疟原虫寄生虫是嘌呤营养缺陷型,仅依赖于人类宿主中预制嘌呤的抢救来提供嘌呤核苷酸的需求。次黄嘌呤-鸟嘌呤-黄嘌呤磷酸核糖基转移酶(HGXPRT)催化6-氧嘌呤碱基向其各自的核苷酸和无机焦磷酸盐的自由可逆Mg 2+依赖性转化。磷酸核糖基衍生自5-磷酸-α- d-核糖基1-焦磷酸酯(PRPP)。由于次黄嘌呤是嘌呤新陈代谢的主要前体,因此来自疟疾寄生虫的酶(Pf HGXPRT)是必不可少的。我们使用了特定的重原子标记在PRPP和次黄嘌呤测定初级(1- 14 C和9- 15N)和次级(1- 3 H和7- 15 N)为固有动力学同位素效应(KIE)值Pf的HGXPRT。内在同位素效应包含用于理解酶促过渡态性质的信息。通过将量子力学计算中预测的KIE值与实验确定的内在值相匹配,探索了Pf HGXPRT的过渡态。此方法提供有关Pf HGXPRT过渡态键长,几何形状和原子电荷分布的信息。Pf的过渡态结构通过克服PRPP的化学不稳定性,在向次黄嘌呤中添加5磷酸核糖的生理方向确定了HGXPRT。为过渡状态Pf的HGXPRT通过发达和近对称d形成核苷酸Ñ * A Ñ,S Ñ 1状过渡状态。



























 京公网安备 11010802027423号
京公网安备 11010802027423号