当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamic Glycosylation Governs the Vertebrate COPII Protein Trafficking Pathway
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-15 00:00:00 , DOI: 10.1021/acs.biochem.7b00870 Nathan J. Cox , Gokhan Unlu 1 , Brittany J. Bisnett , Thomas R. Meister , Brett M. Condon , Peter M. Luo , Timothy J. Smith , Michael Hanna 2 , Abhishek Chhetri , Erik J. Soderblom 3 , Anjon Audhya 2 , Ela W. Knapik 1 , Michael Boyce
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-15 00:00:00 , DOI: 10.1021/acs.biochem.7b00870 Nathan J. Cox , Gokhan Unlu 1 , Brittany J. Bisnett , Thomas R. Meister , Brett M. Condon , Peter M. Luo , Timothy J. Smith , Michael Hanna 2 , Abhishek Chhetri , Erik J. Soderblom 3 , Anjon Audhya 2 , Ela W. Knapik 1 , Michael Boyce
Affiliation
|
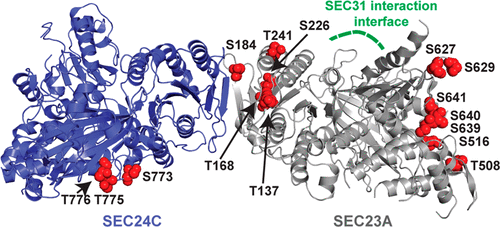
The COPII coat complex, which mediates secretory cargo trafficking from the endoplasmic reticulum, is a key control point for subcellular protein targeting. Because misdirected proteins cannot function, protein sorting by COPII is critical for establishing and maintaining normal cell and tissue homeostasis. Indeed, mutations in COPII genes cause a range of human pathologies, including cranio-lenticulo-sutural dysplasia (CLSD), which is characterized by collagen trafficking defects, craniofacial abnormalities, and skeletal dysmorphology. Detailed knowledge of the COPII pathway is required to understand its role in normal cell physiology and to devise new treatments for disorders in which it is disrupted. However, little is known about how vertebrates dynamically regulate COPII activity in response to developmental, metabolic, or pathological cues. Several COPII proteins are modified by O-linked β-N-acetylglucosamine (O-GlcNAc), a dynamic form of intracellular protein glycosylation, but the biochemical and functional effects of these modifications remain unclear. Here, we use a combination of chemical, biochemical, cellular, and genetic approaches to demonstrate that site-specific O-GlcNAcylation of COPII proteins mediates their protein–protein interactions and modulates cargo secretion. In particular, we show that individual O-GlcNAcylation sites of SEC23A, an essential COPII component, are required for its function in human cells and vertebrate development, because mutation of these sites impairs SEC23A-dependent in vivo collagen trafficking and skeletogenesis in a zebrafish model of CLSD. Our results indicate that O-GlcNAc is a conserved and critical regulatory modification in the vertebrate COPII-dependent trafficking pathway.
中文翻译:

动态糖基化控制脊椎动物COPII蛋白的运输途径。
COPII外壳复合物是介导内质网分泌性货物运输的介质,是亚细胞蛋白靶向的关键控制点。由于方向错误的蛋白质无法发挥功能,因此通过COPII进行蛋白质分选对于建立和维持正常的细胞和组织动态平衡至关重要。确实,COPII基因的突变会引起一系列人类疾病,包括颅-小脑-缝合-异型增生(CLSD),其特征是胶原蛋白运输缺陷,颅面异常和骨骼畸形。需要了解COPII途径的详细知识,以了解其在正常细胞生理学中的作用,并针对其被破坏的疾病设计新的治疗方法。然而,关于脊椎动物如何响应发育,代谢或病理学线索如何动态调节COPII活性知之甚少。N-乙酰氨基葡糖(O-GlcNAc)是细胞内蛋白质糖基化的一种动态形式,但这些修饰的生物化学和功能作用仍不清楚。在这里,我们使用化学,生化,细胞和遗传方法的组合来证明COPII蛋白的位点特异性O-GlcNAcycy介导其蛋白-蛋白相互作用并调节货物分泌。特别是,我们显示出SEC23A的单个O-GlcNAcylation位点(一种必需的COPII组分)对于其在人类细胞和脊椎动物发育中的功能是必需的,因为这些位点的突变会损害体内SEC23A依赖性CLSD的斑马鱼模型中的胶原蛋白运输和骨骼生成。我们的结果表明,O-GlcNAc是脊椎动物COPII依赖性运输途径中的一种保守且至关重要的调节修饰。
更新日期:2017-12-15
中文翻译:

动态糖基化控制脊椎动物COPII蛋白的运输途径。
COPII外壳复合物是介导内质网分泌性货物运输的介质,是亚细胞蛋白靶向的关键控制点。由于方向错误的蛋白质无法发挥功能,因此通过COPII进行蛋白质分选对于建立和维持正常的细胞和组织动态平衡至关重要。确实,COPII基因的突变会引起一系列人类疾病,包括颅-小脑-缝合-异型增生(CLSD),其特征是胶原蛋白运输缺陷,颅面异常和骨骼畸形。需要了解COPII途径的详细知识,以了解其在正常细胞生理学中的作用,并针对其被破坏的疾病设计新的治疗方法。然而,关于脊椎动物如何响应发育,代谢或病理学线索如何动态调节COPII活性知之甚少。N-乙酰氨基葡糖(O-GlcNAc)是细胞内蛋白质糖基化的一种动态形式,但这些修饰的生物化学和功能作用仍不清楚。在这里,我们使用化学,生化,细胞和遗传方法的组合来证明COPII蛋白的位点特异性O-GlcNAcycy介导其蛋白-蛋白相互作用并调节货物分泌。特别是,我们显示出SEC23A的单个O-GlcNAcylation位点(一种必需的COPII组分)对于其在人类细胞和脊椎动物发育中的功能是必需的,因为这些位点的突变会损害体内SEC23A依赖性CLSD的斑马鱼模型中的胶原蛋白运输和骨骼生成。我们的结果表明,O-GlcNAc是脊椎动物COPII依赖性运输途径中的一种保守且至关重要的调节修饰。











































 京公网安备 11010802027423号
京公网安备 11010802027423号