当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Retinal Binding to Apo-Gloeobacter Rhodopsin: The Role of pH and Retinal–Carotenoid Interaction
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acs.jpcb.7b07523 Sankar Jana 1 , Tamar Eliash 1 , Kwang-Hwan Jung 2 , Mordechai Sheves 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acs.jpcb.7b07523 Sankar Jana 1 , Tamar Eliash 1 , Kwang-Hwan Jung 2 , Mordechai Sheves 1
Affiliation
|
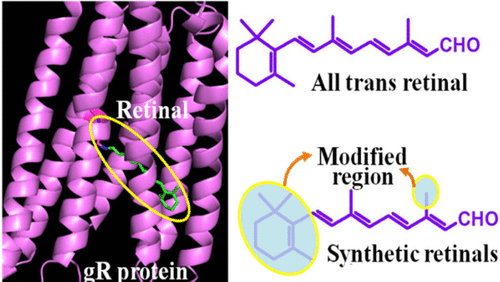
Over the past few decades, the structure, functions, properties, and molecular mechanisms of retinal proteins have been studied extensively. The newly studied retinal protein Gloeobacter rhodopsin (gR) acts as a light-driven proton pump, transferring a proton from the cytoplasmic region to the extracellular region of a cell following light absorption. It was previously shown that gR can bind the carotenoid salinixanthin (sal). In the present study, we report the effect of pH on the binding of retinal to the apo-protein of gR, in the presence and absence of sal, to form the gR pigment. We found that binding at different pH levels reflects the titration of two different protein residues, one at the lower pKa 3.5 and another at the higher pKa 8.4, that affect the pigment’s formation. The maximum amount of pigment was formed at pH 5, both with and without the presence of sal. The introduction of sal accelerates the rate of pigment formation by a factor of 190. Furthermore, it is suggested that occupation of the binding site by the retinal chromophore induces protein conformational alterations which in turn affect the carotenoid conformation, which precedes the formation of the retinal–protein covalent bond. Our examination of synthetic retinal analogues in which the ring structure was modified revealed that, in the absence of sal, the retinal ring structure affects the rate of pigment formation and that the intact structure is needed for efficient pigment formation. However, the presence of sal abolishes this effect, and all-trans retinal and its modified ring analogues bind at a similar rate.
中文翻译:

视网膜与载脂蛋白视紫红质的结合:pH和视网膜-类胡萝卜素相互作用的作用。
在过去的几十年中,已经广泛研究了视网膜蛋白的结构,功能,特性和分子机理。新研究的视网膜蛋白视紫红质视紫红质(gR)充当光驱动的质子泵,在吸收光后将质子从细胞质区域转移到细胞的细胞外区域。先前已证明gR可以结合类胡萝卜素盐黄质(sal)。在本研究中,我们报道了在存在和不存在sal的情况下,pH对视网膜与gR脱辅基蛋白结合形成gR色素的影响。我们发现,在不同pH值下的结合反映了两种不同蛋白质残基的滴定,一个位于较低的p K a 3.5处,另一个位于较高的p K a处。8.4,影响颜料的形成。在有和没有sal的情况下,在pH 5时都形成了最大量的颜料。sal的引入将色素形成的速度加快了190倍。此外,有人提出,视网膜发色团对结合位点的占据会诱导蛋白质构象改变,进而影响类胡萝卜素构象,从而在视网膜形成之前–蛋白共价键。我们对其中环结构被修饰的合成视网膜类似物的检查显示,在没有sal的情况下,视网膜环结构会影响色素形成的速率,而完整的结构是有效形成色素所必需的。但是,sal的存在消除了这种影响,
更新日期:2017-11-22
中文翻译:

视网膜与载脂蛋白视紫红质的结合:pH和视网膜-类胡萝卜素相互作用的作用。
在过去的几十年中,已经广泛研究了视网膜蛋白的结构,功能,特性和分子机理。新研究的视网膜蛋白视紫红质视紫红质(gR)充当光驱动的质子泵,在吸收光后将质子从细胞质区域转移到细胞的细胞外区域。先前已证明gR可以结合类胡萝卜素盐黄质(sal)。在本研究中,我们报道了在存在和不存在sal的情况下,pH对视网膜与gR脱辅基蛋白结合形成gR色素的影响。我们发现,在不同pH值下的结合反映了两种不同蛋白质残基的滴定,一个位于较低的p K a 3.5处,另一个位于较高的p K a处。8.4,影响颜料的形成。在有和没有sal的情况下,在pH 5时都形成了最大量的颜料。sal的引入将色素形成的速度加快了190倍。此外,有人提出,视网膜发色团对结合位点的占据会诱导蛋白质构象改变,进而影响类胡萝卜素构象,从而在视网膜形成之前–蛋白共价键。我们对其中环结构被修饰的合成视网膜类似物的检查显示,在没有sal的情况下,视网膜环结构会影响色素形成的速率,而完整的结构是有效形成色素所必需的。但是,sal的存在消除了这种影响,











































 京公网安备 11010802027423号
京公网安备 11010802027423号