当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Is Solute Rotation in an Ionic Liquid Influenced by the Addition of Glucose?
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acs.jpcb.7b09888 Rajan Maurya 1 , Sudhanshu Naithani 2 , Dibyendu Bandyopadhyay , Niharendu Choudhury , G. B. Dutt
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acs.jpcb.7b09888 Rajan Maurya 1 , Sudhanshu Naithani 2 , Dibyendu Bandyopadhyay , Niharendu Choudhury , G. B. Dutt
Affiliation
|
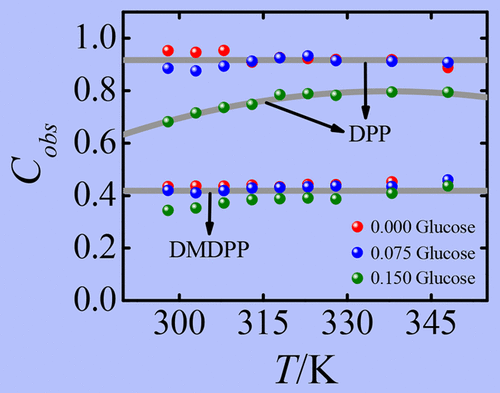
Fluorescence anisotropy measurements and molecular dynamics (MD) simulations have been performed to understand the specific interactions of two structurally similar nondipolar solutes, 2,5-dimethyl-1,4-dioxo-3,6-diphenylpyrrolo[3,4-c]pyrrole (DMDPP) and 1,4-dioxo-3,6-diphenylpyrrolo[3,4-c]pyrrole (DPP), with neat 1-butyl-3-methylimidazolium dicyanamide ([BMIM][N(CN)2]) and also in the presence of glucose. It has been observed that the measured reorientation times of DMDPP in neat [BMIM][N(CN)2] follow the predictions of the Stokes–Einstein–Debye hydrodynamic theory with slip boundary condition. Addition of glucose (0.075 and 0.15 mole fraction) has no bearing on the rotational diffusion of the solute apart from the viscosity related effects. In contrast, the reorientation times of DPP in neat [BMIM][N(CN)2] obey stick boundary condition as the hydrogen bond donating solute experiences specific interactions with the dicyanamide anion. No influence of the additive can be noticed on the rotational diffusion of DPP at 0.075 mole fraction of glucose. However, at 0.15 mole fraction of glucose, the reorientation times of the solute at a given viscosity and temperature decrease by 15–40% compared to those obtained in the neat ionic liquid. MD simulations indicate that each DPP molecule hydrogen bonds with two dicyanamide anions in neat ionic liquid. The simulations also reveal that, at 0.15 mole fraction of glucose, the concentration of anions hydrogen bonded to glucose increases significantly; therefore, the percentage of solute molecules that can form hydrogen bonds with two dicyanamide anions decreases to 84, which leads to faster rotation of DPP.
中文翻译:

离子液体中溶质的旋转受葡萄糖添加的影响吗?
已经进行了荧光各向异性测量和分子动力学(MD)模拟,以了解两种结构相似的非偶极溶质,2,5-二甲基-1,4-二氧代-3,6-二苯基吡咯并[3,4- c ]吡咯的特定相互作用(DMDPP)和1,4-二氧代3,6-二苯基吡咯并[3,4- c ]吡咯(DPP),以及纯净的1-丁基-3-甲基咪唑鎓二氰胺([BMIM] [N(CN)2 ])和在葡萄糖存在下也是如此。已经观察到在纯净的[BMIM] [N(CN)2]遵循带滑移边界条件的Stokes–Einstein–Debye流体力学理论的预测。除了粘度相关的影响外,添加葡萄糖(0.075和0.15摩尔分数)与溶质的旋转扩散无关。相反,纯[BMIM] [N(CN)2服从氢键的溶质与双氰胺阴离子发生特定的相互作用,因此服从棒的边界条件。在葡萄糖的0.075摩尔分数下,没有注意到添加剂对DPP的旋转扩散的影响。但是,在葡萄糖的摩尔分数为0.15时,与纯离子液体相比,在给定的粘度和温度下,溶质的重新定向时间减少了15–40%。MD模拟表明,每个DPP分子在纯离子液体中均与两个双氰胺阴离子形成氢键。模拟还表明,在葡萄糖的摩尔分数为0.15时,与葡萄糖结合的阴离子氢的浓度显着增加。因此,可以与两个双氰胺阴离子形成氢键的溶质分子的百分比降低到84,这导致DPP旋转更快。
更新日期:2017-11-22
中文翻译:

离子液体中溶质的旋转受葡萄糖添加的影响吗?
已经进行了荧光各向异性测量和分子动力学(MD)模拟,以了解两种结构相似的非偶极溶质,2,5-二甲基-1,4-二氧代-3,6-二苯基吡咯并[3,4- c ]吡咯的特定相互作用(DMDPP)和1,4-二氧代3,6-二苯基吡咯并[3,4- c ]吡咯(DPP),以及纯净的1-丁基-3-甲基咪唑鎓二氰胺([BMIM] [N(CN)2 ])和在葡萄糖存在下也是如此。已经观察到在纯净的[BMIM] [N(CN)2]遵循带滑移边界条件的Stokes–Einstein–Debye流体力学理论的预测。除了粘度相关的影响外,添加葡萄糖(0.075和0.15摩尔分数)与溶质的旋转扩散无关。相反,纯[BMIM] [N(CN)2服从氢键的溶质与双氰胺阴离子发生特定的相互作用,因此服从棒的边界条件。在葡萄糖的0.075摩尔分数下,没有注意到添加剂对DPP的旋转扩散的影响。但是,在葡萄糖的摩尔分数为0.15时,与纯离子液体相比,在给定的粘度和温度下,溶质的重新定向时间减少了15–40%。MD模拟表明,每个DPP分子在纯离子液体中均与两个双氰胺阴离子形成氢键。模拟还表明,在葡萄糖的摩尔分数为0.15时,与葡萄糖结合的阴离子氢的浓度显着增加。因此,可以与两个双氰胺阴离子形成氢键的溶质分子的百分比降低到84,这导致DPP旋转更快。



























 京公网安备 11010802027423号
京公网安备 11010802027423号