Immunity ( IF 25.5 ) Pub Date : 2017-11-21 , DOI: 10.1016/j.immuni.2017.10.011 Xavier Clemente-Casares , Siyavash Hosseinzadeh , Iulia Barbu , Sarah A. Dick , Jillian A. Macklin , Yiming Wang , Abdul Momen , Crystal Kantores , Laura Aronoff , Maylis Farno , Tiffany M. Lucas , Joan Avery , Dorrin Zarrin-Khat , Heidi J. Elsaesser , Babak Razani , Kory J. Lavine , Mansoor Husain , David G. Brooks , Clinton S. Robbins , Myron Cybulsky , Slava Epelman
|
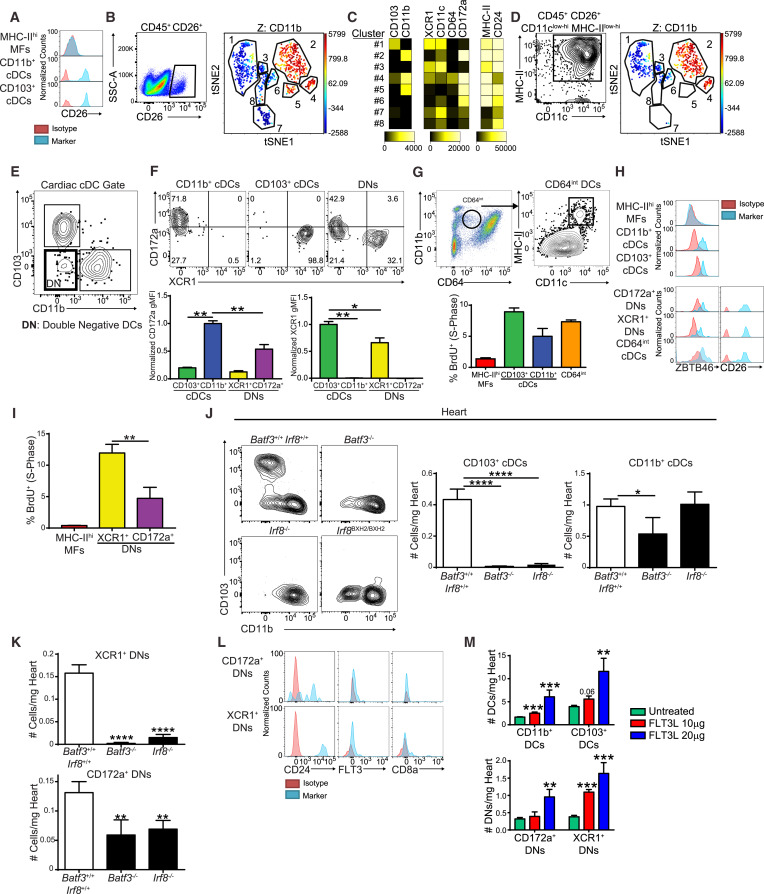
Innate and adaptive immune cells modulate heart failure pathogenesis during viral myocarditis, yet their identities and functions remain poorly defined. We utilized a combination of genetic fate mapping, parabiotic, transcriptional, and functional analyses and demonstrated that the heart contained two major conventional dendritic cell (cDC) subsets, CD103+ and CD11b+, which differentially relied on local proliferation and precursor recruitment to maintain their tissue residency. Following viral infection of the myocardium, cDCs accumulated in the heart coincident with monocyte infiltration and loss of resident reparative embryonic-derived cardiac macrophages. cDC depletion abrogated antigen-specific CD8+ T cell proliferative expansion, transforming subclinical cardiac injury to overt heart failure. These effects were mediated by CD103+ cDCs, which are dependent on the transcription factor BATF3 for their development. Collectively, our findings identified resident cardiac cDC subsets, defined their origins, and revealed an essential role for CD103+ cDCs in antigen-specific T cell responses during subclinical viral myocarditis.
中文翻译:

CD103 +常规树突状细胞监视系统可防止在亚临床病毒性心肌炎期间出现明显的心力衰竭
先天性和适应性免疫细胞可调节病毒性心肌炎期间心力衰竭的发病机制,但其身份和功能仍不清楚。我们结合了遗传命运图谱,共生生物,转录和功能分析,并证明心脏包含两个主要的常规树突状细胞(cDC)亚群CD103 +和CD11b +,它们分别依赖于局部增殖和前体募集来维持它们的功能。组织驻留。心肌病毒感染后,心脏中的cDC积聚在单核细胞浸润和居民修复性胚胎衍生的心脏巨噬细胞丢失的同时。cDC耗竭消除了抗原特异性CD8 +T细胞增殖性扩张,将亚临床心脏损伤转变为明显的心力衰竭。这些作用是由CD103 + cDCs介导的,其依赖于转录因子BATF3的发育。总的来说,我们的发现确定了驻留心脏的cDC亚集,定义了它们的起源,并揭示了CD103 + cDC在亚临床病毒性心肌炎期间在抗原特异性T细胞反应中的重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号