当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Precise Probing of Residue Roles by Post-Translational β,γ-C,N Aza-Michael Mutagenesis in Enzyme Active Sites
ACS Central Science ( IF 18.2 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acscentsci.7b00341 Jitka Dadová 1 , Kuan-Jung Wu 1 , Patrick G. Isenegger 1 , James C. Errey 1 , Gonçalo J. L. Bernardes 1 , Justin M. Chalker 1 , Lluís Raich 2 , Carme Rovira 2, 3 , Benjamin G. Davis 1
ACS Central Science ( IF 18.2 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acscentsci.7b00341 Jitka Dadová 1 , Kuan-Jung Wu 1 , Patrick G. Isenegger 1 , James C. Errey 1 , Gonçalo J. L. Bernardes 1 , Justin M. Chalker 1 , Lluís Raich 2 , Carme Rovira 2, 3 , Benjamin G. Davis 1
Affiliation
|
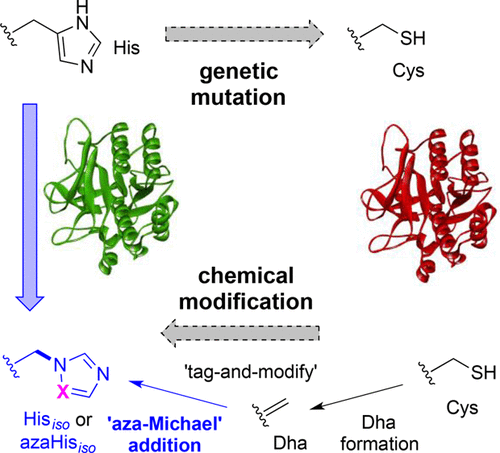
Biomimicry valuably allows the understanding of the essential chemical components required to recapitulate biological function, yet direct strategies for evaluating the roles of amino acids in proteins can be limited by access to suitable, subtly-altered unnatural variants. Here we describe a strategy for dissecting the role of histidine residues in enzyme active sites using unprecedented, chemical, post-translational side-chain-β,γ C–N bond formation. Installation of dehydroalanine (as a “tag”) allowed the testing of nitrogen conjugate nucleophiles in “aza-Michael”-1,4-additions (to “modify”). This allowed the creation of a regioisomer of His (iso-His, Hisiso) linked instead through its pros-Nπ atom rather than naturally linked via C4, as well as an aza-altered variant aza-Hisiso. The site-selective generation of these unnatural amino acids was successfully applied to probe the contributing roles (e.g., size, H-bonding) of His residues toward activity in the model enzymes subtilisin protease from Bacillus lentus and Mycobacterium tuberculosis pantothenate synthetase.
中文翻译:

酶活性位点中翻译后β,γ-C,N Aza-Michael诱变的残基作用的精确探测
仿生学非常有价值地理解了概括生物学功能所必需的基本化学成分,但是通过获得合适的,微妙改变的非天然变体,可以限制用于评估氨基酸在蛋白质中的作用的直接策略。在这里,我们描述了一种策略,该方法使用史无前例的,化学的,翻译后的侧链-β,γC–N键形成来分解组氨酸残基在酶活性位点中的作用。安装脱氢丙氨酸(作为“标签”)可以测试“氮杂-迈克尔” -1,4-加成物中的氮共轭亲核试剂(“修饰”)。这允许创建通过其pros-Nπ原子而非通过C4自然连接的His(iso-His,His iso)的区域异构体,以及aza改变的aza-His iso变体。这些非天然氨基酸的位点选择性生成已成功地用于探究His残基对来自迟缓芽孢杆菌和结核分枝杆菌泛酸合成酶的模型酶枯草杆菌蛋白酶中的活性的贡献作用(例如大小,H键)。
更新日期:2017-11-22
中文翻译:

酶活性位点中翻译后β,γ-C,N Aza-Michael诱变的残基作用的精确探测
仿生学非常有价值地理解了概括生物学功能所必需的基本化学成分,但是通过获得合适的,微妙改变的非天然变体,可以限制用于评估氨基酸在蛋白质中的作用的直接策略。在这里,我们描述了一种策略,该方法使用史无前例的,化学的,翻译后的侧链-β,γC–N键形成来分解组氨酸残基在酶活性位点中的作用。安装脱氢丙氨酸(作为“标签”)可以测试“氮杂-迈克尔” -1,4-加成物中的氮共轭亲核试剂(“修饰”)。这允许创建通过其pros-Nπ原子而非通过C4自然连接的His(iso-His,His iso)的区域异构体,以及aza改变的aza-His iso变体。这些非天然氨基酸的位点选择性生成已成功地用于探究His残基对来自迟缓芽孢杆菌和结核分枝杆菌泛酸合成酶的模型酶枯草杆菌蛋白酶中的活性的贡献作用(例如大小,H键)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号