当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring the Active Center of the LSD1/CoREST Complex by Molecular Dynamics Simulation Utilizing Its Co-crystallized Co-factor Tetrahydrofolate as a Probe
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2017-12-06 00:00:00 , DOI: 10.1021/acs.jcim.7b00256 Waleed A. Zalloum 1 , Hiba M. Zalloum 2
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2017-12-06 00:00:00 , DOI: 10.1021/acs.jcim.7b00256 Waleed A. Zalloum 1 , Hiba M. Zalloum 2
Affiliation
|
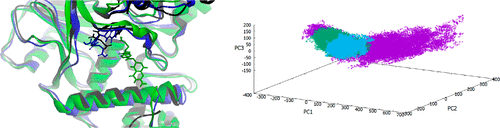
Epigenetic targeting of cancer is a recent effort to manipulate the gene without destroying the genetic material. Lysine-specific demethylase 1 (LSD1) is one of the enzymes associated with the chromatin for post-translational modifications, where it demethylates lysine amino acid in the chromatin H3 tail. Many studies showed that inhibiting LSD1 could potentially be used to treat cancer epigenetically. LSD1 is associated with its corepressor protein CoREST, and it uses tetrahydrofolate as a co-factor to accept CH2 from the demethylation process. In this study, the co-crystallized co-factor tetrahydrofolate was utilized to determine possible binding regions in the active center of the LSD1/CoREST complex. Also, the flexibility of the complex has been investigated by molecular dynamics simulation and subsequent analysis by clustering and principal component analysis. This research supported other studies and showed that LSD1/CoREST complex exists in two main conformational structures: open and closed. Furthermore, this study showed that tetrahydrofolate stably binds to the LSD1/CoREST complex, in its open conformation, at its entrance. It then binds to the core of the complex, inducing the closed conformation. Furthermore, the interactions of tetrahydrofolate to these two binding regions and the corresponding binding mode of tetrahydrofolate were investigated to be used in structure-based drug design.
中文翻译:

以其共结晶的辅因子四氢叶酸为探针,通过分子动力学模拟探索LSD1 / CoREST配合物的活性中心
癌症的表观遗传学靶向是最近在不破坏遗传物质的情况下操纵基因的努力。赖氨酸特异性脱甲基酶1(LSD1)是与染色质相关的酶之一,可进行翻译后修饰,在其中它使染色质H3尾部的赖氨酸氨基酸脱甲基。许多研究表明,抑制LSD1可能被用于表观遗传治疗癌症。LSD1与其共抑制蛋白CoREST相关,并且它使用四氢叶酸作为辅因子来接受CH 2从脱甲基过程。在这项研究中,共结晶的辅因子四氢叶酸被用来确定LSD1 / CoREST复合物活性中心的可能结合区域。同样,已经通过分子动力学模拟以及随后的聚类和主成分分析对复合物的柔性进行了研究。这项研究支持其他研究,并表明LSD1 / CoREST复合物以两个主要构象结构存在:开放和封闭。此外,这项研究表明,四氢叶酸在其入口处以开放构象稳定结合LSD1 / CoREST复合物。然后,它结合到复合物的核心,诱导出封闭的构象。此外,
更新日期:2017-12-06
中文翻译:

以其共结晶的辅因子四氢叶酸为探针,通过分子动力学模拟探索LSD1 / CoREST配合物的活性中心
癌症的表观遗传学靶向是最近在不破坏遗传物质的情况下操纵基因的努力。赖氨酸特异性脱甲基酶1(LSD1)是与染色质相关的酶之一,可进行翻译后修饰,在其中它使染色质H3尾部的赖氨酸氨基酸脱甲基。许多研究表明,抑制LSD1可能被用于表观遗传治疗癌症。LSD1与其共抑制蛋白CoREST相关,并且它使用四氢叶酸作为辅因子来接受CH 2从脱甲基过程。在这项研究中,共结晶的辅因子四氢叶酸被用来确定LSD1 / CoREST复合物活性中心的可能结合区域。同样,已经通过分子动力学模拟以及随后的聚类和主成分分析对复合物的柔性进行了研究。这项研究支持其他研究,并表明LSD1 / CoREST复合物以两个主要构象结构存在:开放和封闭。此外,这项研究表明,四氢叶酸在其入口处以开放构象稳定结合LSD1 / CoREST复合物。然后,它结合到复合物的核心,诱导出封闭的构象。此外,











































 京公网安备 11010802027423号
京公网安备 11010802027423号