当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catholyte Formulations for High-Energy Li–S Batteries
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acs.jpclett.7b02936 Satyajit Phadke 1 , Erwan Coadou 1 , Mérièm Anouti 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acs.jpclett.7b02936 Satyajit Phadke 1 , Erwan Coadou 1 , Mérièm Anouti 1
Affiliation
|
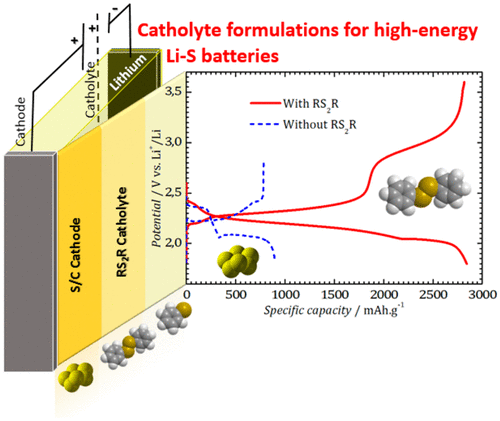
The sulfur electrode in LiS batteries suffers from rapid capacity loss and low efficiency due to the solubility of long chain polysulfides formed during discharge. Herein, we demonstrate the beneficial effect of original catholyte formulations containing redox active organyl disulfides (PhS2Ph) on the capacity utilization and retention as well as the efficiency in LiS batteries. Resulting from the chemical equilibria in the electrolyte between the sulfur/polysulfides (S8/Sx2–) and disulfide/thiolates (PhS2Ph/PhSx–), the polysulfide redox shuttle phenomenon is minimized due to the suppression of formation of soluble polysulfides (Sx2–, x > 4). Using the catholyte containing 0.4 M Ph2S2 as an additive in a standard base electrolyte (DOL/DME + LiTFSI/LiNO3), a stable capacity of 1050 mAh·g–1 is obtained under galvanostatic cycling at C/5 with a Coulombic efficiency of >99.5%. At 45 °C, it is shown that the formulated catholyte enables galvanostatic cycling at a high c-rate of 1C over 500 cycles with a capacity above 900 mAh·g–1 and a high energy efficiency of 82%.
中文翻译:

高能锂电池的阴极电解液配方
LiS电池中的硫电极由于在放电过程中形成的长链多硫化物的溶解性而导致快速的容量损失和低效率。在此,我们证明了含有氧化还原活性有机二硫化物(PhS 2 Ph)的原始阴极电解液配方对LiS电池的容量利用率和保持率以及效率的有益影响。由于硫/多硫化物(S 8 / S x 2–)和二硫化物/硫醇盐(PhS 2 Ph / PhS x –)之间电解质中的化学平衡,多硫化物氧化还原穿梭现象被抑制到最小。可溶性多硫化物(S x 2–,x > 4)。在标准基础电解质(DOL / DME + LiTFSI / LiNO 3)中,使用含有0.4 M Ph 2 S 2的阴极电解液作为添加剂,在C / 5的恒电流循环下,在3/4的恒电流下可获得1050 mAh·g –1的稳定容量。库仑效率> 99.5%。结果表明,在45°C时,配制的阴极电解液可以在500个循环中以1 C的高c速率进行恒电流循环,容量超过900 mAh·g –1,能量效率高达82%。
更新日期:2017-11-22
中文翻译:

高能锂电池的阴极电解液配方
LiS电池中的硫电极由于在放电过程中形成的长链多硫化物的溶解性而导致快速的容量损失和低效率。在此,我们证明了含有氧化还原活性有机二硫化物(PhS 2 Ph)的原始阴极电解液配方对LiS电池的容量利用率和保持率以及效率的有益影响。由于硫/多硫化物(S 8 / S x 2–)和二硫化物/硫醇盐(PhS 2 Ph / PhS x –)之间电解质中的化学平衡,多硫化物氧化还原穿梭现象被抑制到最小。可溶性多硫化物(S x 2–,x > 4)。在标准基础电解质(DOL / DME + LiTFSI / LiNO 3)中,使用含有0.4 M Ph 2 S 2的阴极电解液作为添加剂,在C / 5的恒电流循环下,在3/4的恒电流下可获得1050 mAh·g –1的稳定容量。库仑效率> 99.5%。结果表明,在45°C时,配制的阴极电解液可以在500个循环中以1 C的高c速率进行恒电流循环,容量超过900 mAh·g –1,能量效率高达82%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号