Catalysis Today ( IF 5.2 ) Pub Date : 2017-11-21 , DOI: 10.1016/j.cattod.2017.11.021 Hidetaka Nanao , Hiroki Sasaki , Osamu Sato , Aritomo Yamaguchi , Masayuki Shirai
|
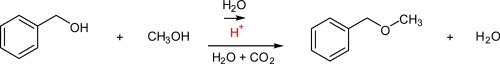
Synthesis of benzyl methyl ether from benzyl alcohol and methanol in high-temperature carbonic water was studied in a batch reactor. Benzyl methyl ether formation was not observed by reacting benzyl alcohol with only methanol under supercritical conditions at 573 K. On the other hand, benzyl methyl ether was formed by the treatment of benzyl alcohol in an aqueous methanol solution at 573 K. 12% of benzyl methyl ether yield was obtained in aqueous methanol solution (methanol to water molar ratio of 0.025 mol/0.11 mol) at 573 K in 60 min and the yield enhanced to 33% by the addition of 18 MPa of carbon dioxide to the aqueous solution. As the etherification is an acid catalyzed reaction, the protons derived from the dissociation of water molecules could be responsible for the etherification of benzyl alcohol in an aqueous methanol solution at 573 K. The enhancement of benzyl methyl ether yield by the addition of carbon dioxide in aqueous methanol solution is caused by the increase of the number of protons derived from carbonic acid, which is formed in high-temperature liquid water under high-pressured carbon dioxide.
中文翻译:

在碳酸水中由苯甲醇和甲醇生产苄基甲基醚
在间歇反应器中研究了由苄醇和甲醇在高温碳酸水中合成苄基甲基醚。苄基醇仅在573 K的超临界条件下仅与甲醇反应,未观察到苄基甲基醚的形成。另一方面,苄基甲基醚是通过在573 K的甲醇水溶液中处理苄醇而形成的。在60分钟内在573 K的甲醇水溶液(甲醇与水的摩尔比为0.025 mol / 0.11 mol)中获得甲醚收率,并通过向水溶液中添加18 MPa的二氧化碳将收率提高至33%。由于醚化反应是酸催化的反应,因此水分子解离产生的质子可能会导致在573 K的甲醇水溶液中苯甲醇的醚化反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号