当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, Characterization, and Polymerization of Fused-Ring Naphthoxazine Resins
Macromolecules ( IF 5.1 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1021/acs.macromol.7b00932 Carlos R. Arza 1 , Pablo Froimowicz 1, 2 , Lu Han 1 , Robert Graf 3 , Hatsuo Ishida 1
Macromolecules ( IF 5.1 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1021/acs.macromol.7b00932 Carlos R. Arza 1 , Pablo Froimowicz 1, 2 , Lu Han 1 , Robert Graf 3 , Hatsuo Ishida 1
Affiliation
|
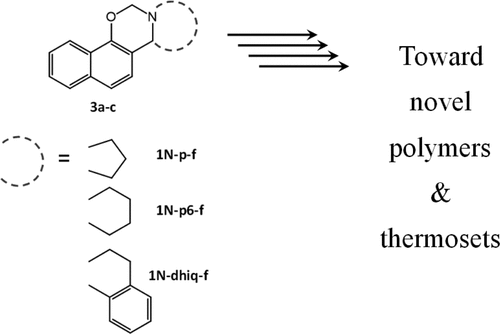
Fused-ring naphthoxazine monomers are synthesized from 1-naphthols and cycloimines derived from piperidine and pyrrolidine. Their polymerization has been confirmed by differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and Fourier transform infrared spectroscopy (FT-IR) as well as by solution and solid-state 1H and 13C nuclear magnetic resonance spectroscopy (NMR). A polymerization reaction similar to the traditional benzoxazine monomers is found with oxazine ring disappearing and hydroxyl group forming by the nonisothermal FT-IR experiment. Fused-ring monomers, studied in this work, polymerize at significantly lower temperatures compared to the ordinary benzoxazine resins.
中文翻译:

稠环萘并恶嗪树脂的设计,合成,表征和聚合
稠环萘并恶嗪单体是由1-萘酚和衍生自哌啶和吡咯烷的环亚胺合成的。通过差示扫描量热法(DSC),热重分析(TGA)和傅里叶变换红外光谱(FT-IR)以及溶液和固态1 H和13 C核磁共振光谱(NMR)证实了它们的聚合。。通过非等温FT-IR实验发现与传统的苯并恶嗪单体相似的聚合反应具有恶嗪环消失和羟基形成。与普通的苯并恶嗪树脂相比,在这项工作中研究的稠环单体在较低的温度下聚合。
更新日期:2017-11-20
中文翻译:

稠环萘并恶嗪树脂的设计,合成,表征和聚合
稠环萘并恶嗪单体是由1-萘酚和衍生自哌啶和吡咯烷的环亚胺合成的。通过差示扫描量热法(DSC),热重分析(TGA)和傅里叶变换红外光谱(FT-IR)以及溶液和固态1 H和13 C核磁共振光谱(NMR)证实了它们的聚合。。通过非等温FT-IR实验发现与传统的苯并恶嗪单体相似的聚合反应具有恶嗪环消失和羟基形成。与普通的苯并恶嗪树脂相比,在这项工作中研究的稠环单体在较低的温度下聚合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号