当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility Model for Ferrous Iron Hydroxide, Hibbingite, Siderite, and Chukanovite in High Saline Solutions of Sodium Chloride, Sodium Sulfate, and Sodium Carbonate
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00065 Sungtae Kim 1 , Cassandra Marrs 1 , Martin Nemer 1 , Jay Je-Hun Jang 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00065 Sungtae Kim 1 , Cassandra Marrs 1 , Martin Nemer 1 , Jay Je-Hun Jang 1
Affiliation
|
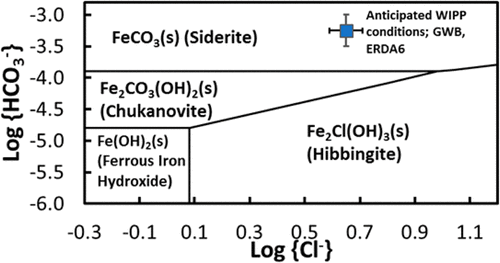
A solubility model is presented for ferrous iron hydroxide (Fe(OH)2(s)), hibbingite (Fe2Cl(OH)3(s)), siderite (FeCO3(s)), and chukanovite (Fe2CO3(OH)2(s)). The Pitzer activity coefficient equation was utilized in developing the model to account for the excess free energies of aqueous species in the background solutions of high ionic strength. Solubility limiting minerals were analyzed before and after experiments using X-ray diffraction. Formation of Fe(OH)2(s) was observed in the experiments that were initiated with Fe2Cl(OH)3(s) in Na2SO4 solution. Coexistence of siderite and chukanovite was observed in the experiments in Na2CO3 + NaCl solutions. Two equilibrium constants that had been reported by us for the dissolution of Fe(OH)2(s) and Fe2Cl(OH)3(s) (Nemer et al.) were rederived in this paper, using newer thermodynamic data selected from the literature to maintain internal consistency of the series of our data analyses in preparation, including this paper. Three additional equilibrium constants for the following reactions were determined in this paper: dissolution of siderite and chukanovite and dissociation of the aqueous species Fe(CO3)2–2. Five Pitzer interaction parameters were derived in this paper: β(0), β(1), and Cφ parameters for the species pair Fe+2/SO4–2; β(0) and β(1) parameters for the species pair Na+/Fe(CO3)2–2. Our model predicts that, among the four inorganic ferrous iron minerals, siderite is the stable mineral in two WIPP-related brines (WIPP: Waste Isolation Pilot Plant), i.e., GWB and ERDA6 (Brush and Domski), and the electrochemical equilibrium between elemental iron and siderite provides a low oxygen fugacity (10–91.2 atm) that can keep the actinides at their lowest oxidation states. (Nemer et al., Brush and Domski; references numbered 1 and 2 in the main text).
中文翻译:

氯化亚铁,硫酸钠和碳酸钠高盐溶液中的氢氧化铁亚铁,菱铁矿,菱铁矿和chukanovite的溶解度模型
提出了一种溶解性模型,用于氢氧化亚铁(Fe(OH)2(s)),菱铁矿(Fe 2 Cl(OH)3(s)),菱铁矿(FeCO 3(s))和chukanovite(Fe 2 CO 3(OH)2(s))。Pitzer活度系数方程式用于开发模型,以说明高离子强度背景溶液中水性物质的过量自由能。在使用X射线衍射进行实验之前和之后,对溶解度限制矿物进行了分析。在Na 2 SO 4中以Fe 2 Cl(OH)3(s)引发的实验中观察到了Fe(OH)2(s)的形成。解决方案。在Na 2 CO 3 + NaCl溶液中的实验中观察到菱铁矿和chukanovite的共存。本文使用选自以下的较新的热力学数据重新得出了我们报道的关于Fe(OH)2(s)和Fe 2 Cl(OH)3(s)溶解的两个平衡常数(Nemer等人)。为保持内部一致性的文献,我们在准备包括本文在内的一系列数据分析时。本文确定了以下反应的三个附加平衡常数:菱铁矿和chukanovite的溶解和水物种Fe(CO 3)2 –2的解离。五匹兹相互作用参数,推导出在本文中:β (0),β (1) ,和Ç φ为物种参数配对的Fe 2 / SO 4 -2 ; Na + / Fe(CO 3)2 –2物种对的β (0)和β (1)参数。我们的模型预测,在四种无机亚铁矿物中,菱铁矿是两种与WIPP相关的盐水(WIPP:废物隔离中试工厂)中的稳定矿物,即GWB和ERDA6(Brush和Domski),元素之间的电化学平衡铁和菱铁矿的氧逸度低(10 –91.2atm)可以使the系元素保持在最低的氧化态。(Nemer等人,Brush和Domski;在正文中引用的编号为1和2)。
更新日期:2017-11-20
中文翻译:

氯化亚铁,硫酸钠和碳酸钠高盐溶液中的氢氧化铁亚铁,菱铁矿,菱铁矿和chukanovite的溶解度模型
提出了一种溶解性模型,用于氢氧化亚铁(Fe(OH)2(s)),菱铁矿(Fe 2 Cl(OH)3(s)),菱铁矿(FeCO 3(s))和chukanovite(Fe 2 CO 3(OH)2(s))。Pitzer活度系数方程式用于开发模型,以说明高离子强度背景溶液中水性物质的过量自由能。在使用X射线衍射进行实验之前和之后,对溶解度限制矿物进行了分析。在Na 2 SO 4中以Fe 2 Cl(OH)3(s)引发的实验中观察到了Fe(OH)2(s)的形成。解决方案。在Na 2 CO 3 + NaCl溶液中的实验中观察到菱铁矿和chukanovite的共存。本文使用选自以下的较新的热力学数据重新得出了我们报道的关于Fe(OH)2(s)和Fe 2 Cl(OH)3(s)溶解的两个平衡常数(Nemer等人)。为保持内部一致性的文献,我们在准备包括本文在内的一系列数据分析时。本文确定了以下反应的三个附加平衡常数:菱铁矿和chukanovite的溶解和水物种Fe(CO 3)2 –2的解离。五匹兹相互作用参数,推导出在本文中:β (0),β (1) ,和Ç φ为物种参数配对的Fe 2 / SO 4 -2 ; Na + / Fe(CO 3)2 –2物种对的β (0)和β (1)参数。我们的模型预测,在四种无机亚铁矿物中,菱铁矿是两种与WIPP相关的盐水(WIPP:废物隔离中试工厂)中的稳定矿物,即GWB和ERDA6(Brush和Domski),元素之间的电化学平衡铁和菱铁矿的氧逸度低(10 –91.2atm)可以使the系元素保持在最低的氧化态。(Nemer等人,Brush和Domski;在正文中引用的编号为1和2)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号