当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Long-Range Organization of Membrane-Curving Proteins
ACS Central Science ( IF 12.7 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acscentsci.7b00392 Mijo Simunovic 1 , Anđela Šarić 2 , J Michael Henderson 1 , Ka Yee C Lee 1 , Gregory A Voth 1
ACS Central Science ( IF 12.7 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acscentsci.7b00392 Mijo Simunovic 1 , Anđela Šarić 2 , J Michael Henderson 1 , Ka Yee C Lee 1 , Gregory A Voth 1
Affiliation
|
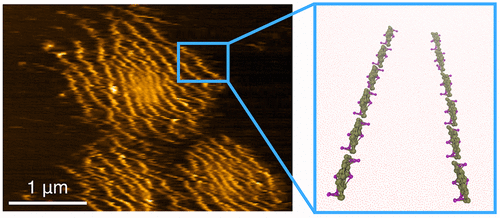
Biological membranes have a central role in mediating the organization of membrane-curving proteins, a dynamic process that has proven to be challenging to probe experimentally. Using atomic force microscopy, we capture the hierarchically organized assemblies of Bin/amphiphysin/Rvs (BAR) proteins on supported lipid membranes. Their structure reveals distinct long linear aggregates of proteins, regularly spaced by up to 300 nm. Employing accurate free-energy calculations from large-scale coarse-grained computer simulations, we found that the membrane mediates the interaction among protein filaments as a combination of short- and long-ranged interactions. The long-ranged component acts at strikingly long distances, giving rise to a variety of micron-sized ordered patterns. This mechanism may contribute to the long-ranged spatiotemporal control of membrane remodeling by proteins in the cell.
中文翻译:

膜弯曲蛋白的长程组织
生物膜在介导膜弯曲蛋白的组织方面发挥着核心作用,这一动态过程已被证明在实验上探测起来具有挑战性。使用原子力显微镜,我们捕获了支持脂质膜上的 Bin/amphiphyn/Rvs (BAR) 蛋白的分层组织组装。它们的结构揭示了蛋白质的明显长线性聚集体,规则间隔高达 300 nm。利用大规模粗粒度计算机模拟的精确自由能计算,我们发现膜以短程和长程相互作用的组合介导蛋白质丝之间的相互作用。长程成分的作用距离惊人,产生各种微米级的有序图案。这种机制可能有助于细胞中蛋白质对膜重塑的远程时空控制。
更新日期:2017-11-21
中文翻译:

膜弯曲蛋白的长程组织
生物膜在介导膜弯曲蛋白的组织方面发挥着核心作用,这一动态过程已被证明在实验上探测起来具有挑战性。使用原子力显微镜,我们捕获了支持脂质膜上的 Bin/amphiphyn/Rvs (BAR) 蛋白的分层组织组装。它们的结构揭示了蛋白质的明显长线性聚集体,规则间隔高达 300 nm。利用大规模粗粒度计算机模拟的精确自由能计算,我们发现膜以短程和长程相互作用的组合介导蛋白质丝之间的相互作用。长程成分的作用距离惊人,产生各种微米级的有序图案。这种机制可能有助于细胞中蛋白质对膜重塑的远程时空控制。










































 京公网安备 11010802027423号
京公网安备 11010802027423号