当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of a Novel Series of Tankyrase Inhibitors by a Hybridization Approach
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00883 Upendra Rao Anumala 1 , Jo Waaler 2, 3 , Yves Nkizinkiko 4 , Alexander Ignatev 4 , Katina Lazarow 1 , Peter Lindemann 1 , Petter Angell Olsen 2, 3 , Sudarshan Murthy 4 , Ezeogo Obaji 4 , Alexander G. Majouga 5 , Sergey Leonov 6, 7 , Jens Peter von Kries 1, 8 , Lari Lehtiö 4 , Stefan Krauss 2, 3 , Marc Nazaré 1, 8
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00883 Upendra Rao Anumala 1 , Jo Waaler 2, 3 , Yves Nkizinkiko 4 , Alexander Ignatev 4 , Katina Lazarow 1 , Peter Lindemann 1 , Petter Angell Olsen 2, 3 , Sudarshan Murthy 4 , Ezeogo Obaji 4 , Alexander G. Majouga 5 , Sergey Leonov 6, 7 , Jens Peter von Kries 1, 8 , Lari Lehtiö 4 , Stefan Krauss 2, 3 , Marc Nazaré 1, 8
Affiliation
|
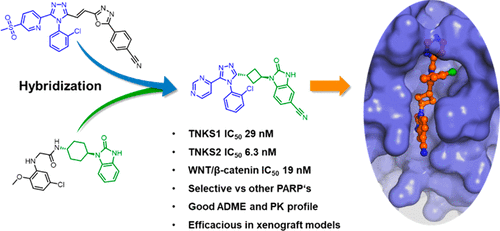
A structure-guided hybridization approach using two privileged substructures gave instant access to a new series of tankyrase inhibitors. The identified inhibitor 16 displays high target affinity on tankyrase 1 and 2 with biochemical and cellular IC50 values of 29 nM, 6.3 nM and 19 nM, respectively, and high selectivity toward other poly (ADP-ribose) polymerase enzymes. The identified inhibitor shows a favorable in vitro ADME profile as well as good oral bioavailability in mice, rats, and dogs. Critical for the approach was the utilization of an appropriate linker between 1,2,4-triazole and benzimidazolone moieties, whereby a cyclobutyl linker displayed superior affinity compared to a cyclohexane and phenyl linker.
中文翻译:

通过杂交方法发现一系列新颖的坦科聚合酶抑制剂
使用两个特权子结构的结构指导杂交方法可立即获得一系列新的tankyrase抑制剂。鉴定出的抑制剂16对tankyrase 1和2表现出较高的靶亲和力,其生化和细胞IC 50值分别为29 nM,6.3 nM和19 nM,并且对其他聚ADP-核糖聚合酶的选择性高。鉴定出的抑制剂在小鼠,大鼠和狗中显示出良好的体外ADME活性以及良好的口服生物利用度。该方法的关键是在1,2,4-三唑和苯并咪唑酮部分之间使用适当的连接基,与环己烷和苯基连接基相比,环丁基连接基显示出优异的亲和力。
更新日期:2017-11-20
中文翻译:

通过杂交方法发现一系列新颖的坦科聚合酶抑制剂
使用两个特权子结构的结构指导杂交方法可立即获得一系列新的tankyrase抑制剂。鉴定出的抑制剂16对tankyrase 1和2表现出较高的靶亲和力,其生化和细胞IC 50值分别为29 nM,6.3 nM和19 nM,并且对其他聚ADP-核糖聚合酶的选择性高。鉴定出的抑制剂在小鼠,大鼠和狗中显示出良好的体外ADME活性以及良好的口服生物利用度。该方法的关键是在1,2,4-三唑和苯并咪唑酮部分之间使用适当的连接基,与环己烷和苯基连接基相比,环丁基连接基显示出优异的亲和力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号