当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ni-Catalyzed cross-coupling reactions of N-acylpyrrole-type amides with organoboron reagents
Chemical Communications ( IF 4.9 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1039/c7cc07457c Pei-Qiang Huang 1, 2, 3, 4, 5 , Hang Chen 1, 2, 3, 4, 5
Chemical Communications ( IF 4.9 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1039/c7cc07457c Pei-Qiang Huang 1, 2, 3, 4, 5 , Hang Chen 1, 2, 3, 4, 5
Affiliation
|
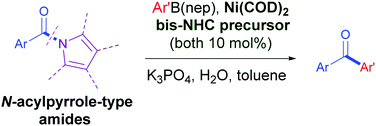
The catalytic conversion of amides to ketones is highly desirable yet challenging in organic synthesis. We herein report the first Ni/bis-NHC-catalyzed cross-coupling of N-acylpyrrole-type amides with arylboronic esters to obtain diarylketones. This method is facilitated by a new chelating bis-NHC ligand. The reaction tolerates diverse functional groups on both arylamide and arylboronic ester partners including sensitive ester and ketone groups.
中文翻译:

Ni- N-酰基吡咯型酰胺与有机硼试剂的镍催化交叉偶联反应
酰胺向酮的催化转化是非常需要的,但是在有机合成中具有挑战性。我们在此报道了N-酰基吡咯型酰胺与芳基硼酸酯的第一次Ni /双-NHC催化的交叉偶联,以获得二芳基酮。新的螯合双-NHC配体促进了该方法。该反应耐受芳基酰胺和芳基硼酸酯伙伴上的各种官能团,包括敏感的酯基和酮基。
更新日期:2017-11-21
中文翻译:

Ni- N-酰基吡咯型酰胺与有机硼试剂的镍催化交叉偶联反应
酰胺向酮的催化转化是非常需要的,但是在有机合成中具有挑战性。我们在此报道了N-酰基吡咯型酰胺与芳基硼酸酯的第一次Ni /双-NHC催化的交叉偶联,以获得二芳基酮。新的螯合双-NHC配体促进了该方法。该反应耐受芳基酰胺和芳基硼酸酯伙伴上的各种官能团,包括敏感的酯基和酮基。



























 京公网安备 11010802027423号
京公网安备 11010802027423号