当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fluorogenic diazaborine formation of semicarbazide with designed coumarin derivatives
Chemical Communications ( IF 4.3 ) Pub Date : 2017-10-24 00:00:00 , DOI: 10.1039/c7cc07389e Samantha Cambray 1, 2, 3, 4, 5 , Anupam Bandyopadhyay 1, 2, 3, 4, 5 , Jianmin Gao 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2017-10-24 00:00:00 , DOI: 10.1039/c7cc07389e Samantha Cambray 1, 2, 3, 4, 5 , Anupam Bandyopadhyay 1, 2, 3, 4, 5 , Jianmin Gao 1, 2, 3, 4, 5
Affiliation
|
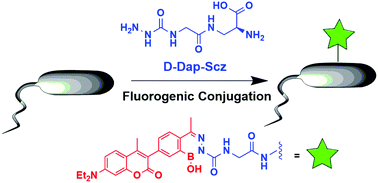
Bioorthogonal fluorogenic reactions serve as enabling tools in research and biotechnology. Herein we describe fluorogenic conjugations of semicarbazide with coumarin derivatives that incorporate a 2-acetylphenylboronic acid motif. These designed coumarins rapidly conjugate with semicarbazide to give diazaborine products with significantly enhanced fluorescence. To demonstrate potential applications of this fluorogenic reaction, we synthesized a semicarbazide-presenting amino acid D-Dap-Scz, which readily incorporates into the cell wall of Staphalococcus aureus and serves as a handle for conjugation with the coumarins. The fluorogenic conjugation of the coumarins to cell surface semicarbazide enables facile visualization of D-Dap-Scz treated bacteria.
中文翻译:

用设计的香豆素衍生物形成氨基脲的氟二氮杂硼嗪形成
正交的荧光反应是研究和生物技术中的辅助工具。在本文中,我们描述了氨基脲与结合了2-乙酰基苯基硼酸基序的香豆素衍生物的荧光共轭。这些设计的香豆素可与氨基脲快速缀合,以提供具有显着增强的荧光的重氮唑啉产品。为了证明该发荧光反应的潜在应用,我们合成了呈现氨基脲的氨基酸D -Dap-Scz,该氨基酸易于并入金黄色葡萄球菌的细胞壁,并用作与香豆素结合的手柄。香豆素与细胞表面半脲的荧光缀合使D -Dap-Scz处理的细菌易于观察。
更新日期:2017-11-21
中文翻译:

用设计的香豆素衍生物形成氨基脲的氟二氮杂硼嗪形成
正交的荧光反应是研究和生物技术中的辅助工具。在本文中,我们描述了氨基脲与结合了2-乙酰基苯基硼酸基序的香豆素衍生物的荧光共轭。这些设计的香豆素可与氨基脲快速缀合,以提供具有显着增强的荧光的重氮唑啉产品。为了证明该发荧光反应的潜在应用,我们合成了呈现氨基脲的氨基酸D -Dap-Scz,该氨基酸易于并入金黄色葡萄球菌的细胞壁,并用作与香豆素结合的手柄。香豆素与细胞表面半脲的荧光缀合使D -Dap-Scz处理的细菌易于观察。











































 京公网安备 11010802027423号
京公网安备 11010802027423号