当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Excimer Formation Dynamics of Dipyrenyldecane in Structurally Different Ionic Liquids
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acs.jpcb.7b08329 Anita Yadav 1 , Siddharth Pandey 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acs.jpcb.7b08329 Anita Yadav 1 , Siddharth Pandey 1
Affiliation
|
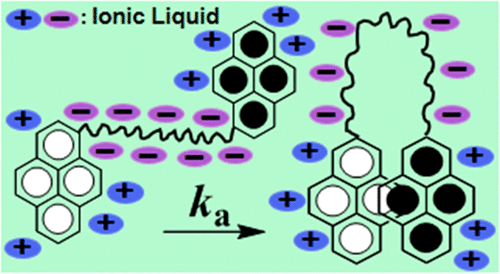
Ionic liquids, being composed of ions alone, may offer alternative pathways for molecular aggregation. These pathways could be controlled by the chemical structure of the cation and the anion of the ionic liquids. Intramolecular excimer formation dynamics of a bifluorophoric probe, 1,3-bis(1-pyrenyl)decane [1Py(10)1Py], where the fluorophoric pyrene moieties are separated by a long decyl chain, is investigated in seven different ionic liquids in 10–90 °C temperature range. The long alkyl separator allows for ample interaction with the solubilizing milieu prior to the formation of the excimer. The ionic liquids are composed of two sets, one having four ionic liquids of 1-butyl-3-methylimidazolium cation ([bmim+]) with different anions and the other having four ionic liquids of bis(trifluoromethylsulfonyl)imide anion ([Tf2N–]) with different cations. The excimer-to-monomer emission intensity ratio (IE/IM) is found to increase with increasing temperature in sigmoidal fashion. Chemical structure of the ionic liquid controls the excimer formation efficiency, as IE/IM values within ionic liquids with the same viscosities are found to be significantly different. The excited-state intensity decay kinetics of 1Py(10)1Py in ionic liquids do not adhere to a simplistic Birk’s scheme, where only one excimer conformer forms after excitation. The apparent rate constants of excimer formation (ka) in highly viscous ionic liquids are an order of magnitude lower than those reported in organic solvents. In general, the higher the viscosity of the ionic liquid, the more sensitive is the ka to the temperature with higher activation energy, Ea. The trend in Ea is found to be similar to that for activation energy of the viscous flow (Ea,η). Stokes–Einstein relationship is not followed in [bmim+] ionic liquids; however, with the exception of [choline][Tf2N], it is found to be followed in [Tf2N–] ionic liquids suggesting the cyclization dynamics of 1Py(10)1Py to be diffusion-controlled and to depend on the viscosity of the ionic liquid irrespective of the identity of the cation. The dependence of ionic liquid structure on cyclization dynamics to form intramolecular excimer is amply highlighted.
中文翻译:

结构不同的离子液体中二苯甲基癸烷的准分子形成动力学
仅由离子组成的离子液体可为分子聚集提供其他途径。这些途径可以通过阳离子的化学结构和离子液体的阴离子来控制。在10种七种不同的离子液体中研究了双荧光探针1,3-双(1-吡啶基)癸烷[1Py(10)1Py]的分子内准分子形成动力学,其中荧光phor部分被长的癸基链隔开。 –90°C温度范围。长烷基分隔物允许在形成受激准分子之前与增溶环境充分相互作用。离子液体由两组组成,一组具有1-丁基-3-甲基咪唑鎓阳离子([bmim +])带有不同的阴离子,而另一种则有四种具有不同阳离子的双(三氟甲基磺酰基)酰亚胺阴离子([Tf 2 N – ])离子液体。发现准分子与单体的发射强度比(I E / I M)随着温度的升高以S形增加。离子液体的化学结构控制准分子形成效率,因为I E / I M发现具有相同粘度的离子液体中的值显着不同。离子液体中1Py(10)1Py的激发态强度衰减动力学不遵循简单的Birk方案,该方案在激发后仅形成一个准分子构象体。受激准分子形成(的表观速率常数ķ一个在高粘度的离子液体)是一个数量级比在有机溶剂中报道降低。通常,离子液体的粘度越高,越敏感是ķ一个以更高的活化能,温度Ë一个。发现E a的趋势与粘性流的活化能(Ea,η)。[bmim + ]离子液体中未遵循Stokes-Einstein关系;但是,除了[胆碱] [Tf 2 N]以外,在[Tf 2 N – ]离子液体中也发现了这种现象,这表明1Py(10)1Py的环化动力学受扩散控制并取决于离子液体的粘度与阳离子的身份无关。充分强调了离子液体结构对形成分子内准分子的环化动力学的依赖性。
更新日期:2017-11-21
中文翻译:

结构不同的离子液体中二苯甲基癸烷的准分子形成动力学
仅由离子组成的离子液体可为分子聚集提供其他途径。这些途径可以通过阳离子的化学结构和离子液体的阴离子来控制。在10种七种不同的离子液体中研究了双荧光探针1,3-双(1-吡啶基)癸烷[1Py(10)1Py]的分子内准分子形成动力学,其中荧光phor部分被长的癸基链隔开。 –90°C温度范围。长烷基分隔物允许在形成受激准分子之前与增溶环境充分相互作用。离子液体由两组组成,一组具有1-丁基-3-甲基咪唑鎓阳离子([bmim +])带有不同的阴离子,而另一种则有四种具有不同阳离子的双(三氟甲基磺酰基)酰亚胺阴离子([Tf 2 N – ])离子液体。发现准分子与单体的发射强度比(I E / I M)随着温度的升高以S形增加。离子液体的化学结构控制准分子形成效率,因为I E / I M发现具有相同粘度的离子液体中的值显着不同。离子液体中1Py(10)1Py的激发态强度衰减动力学不遵循简单的Birk方案,该方案在激发后仅形成一个准分子构象体。受激准分子形成(的表观速率常数ķ一个在高粘度的离子液体)是一个数量级比在有机溶剂中报道降低。通常,离子液体的粘度越高,越敏感是ķ一个以更高的活化能,温度Ë一个。发现E a的趋势与粘性流的活化能(Ea,η)。[bmim + ]离子液体中未遵循Stokes-Einstein关系;但是,除了[胆碱] [Tf 2 N]以外,在[Tf 2 N – ]离子液体中也发现了这种现象,这表明1Py(10)1Py的环化动力学受扩散控制并取决于离子液体的粘度与阳离子的身份无关。充分强调了离子液体结构对形成分子内准分子的环化动力学的依赖性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号