当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Proton-Coupled Electron Transfer and Substituent Effects in Catechol-Based Deep Eutectic Solvents: Gross and Fine Tuning of Redox Activity
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1021/acs.jpcb.7b10169 Parker J. Smith 1 , John C. Goeltz 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1021/acs.jpcb.7b10169 Parker J. Smith 1 , John C. Goeltz 1
Affiliation
|
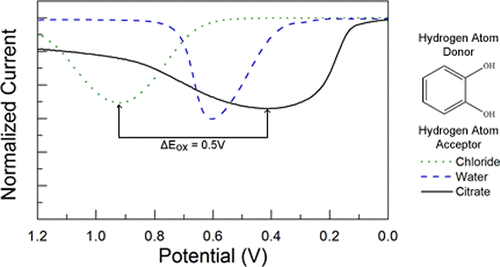
The 1,2-diol moiety in a variety of substituted catechols allows formation of room temperature ionic melts in a 2:1 ratio with choline chloride or choline dihydrogen citrate. These deep eutectic solvents were 4.3–6.6 M in redox active catechols. Substituents on 3- and 4-substituted catechols shift both E° and pKa such that Hammett parameters predict the observed Ep for oxidation in square wave voltammetry. The proton acceptor for the proton-coupled oxidation shifts the observed Ep more strongly than the substituents within the substituents and acceptors reported here. The shift is predicted well by the pKa of the conjugate acid of the proton acceptor, i.e., water in aqueous solutions or chloride or dihydrogen citrate in the DESs in this study. Together, the substituent and the proton acceptor allow gross and fine-tuning of the oxidation potential for catechol over 750 mV, the first demonstration of control of the thermodynamics of proton-coupled electron transfer in deep eutectic solvents. Changing the substituents on the HBD affords fine control in tens of millivolts, while changing the base strength of the anion of the organic salt affords gross control across hundreds of millivolts.
中文翻译:

基于邻苯二酚的深共熔溶剂中的质子耦合电子转移和取代基效应:氧化还原活性的总体和精细调整
各种取代的邻苯二酚中的1,2-二醇部分允许与氯化胆碱或柠檬酸胆碱二氢盐以2:1的比例形成室温离子熔体。这些深度共晶溶剂在氧化还原活性邻苯二酚中为4.3–6.6M。3-和4-取代的邻苯二酚上的取代基使E °和p K a都发生位移,以使Hammett参数预测在方波伏安法中观察到的E p对氧化的作用。用于质子偶联氧化的质子受体比在此报道的取代基和受体中的取代基更强烈地移动观察到的E p。通过p K a可以很好地预测该变化本研究中质子受体的共轭酸,即水溶液中的水或DES中的氯化物或柠檬酸二氢盐的含量。取代基和质子受体共同作用,可对750 mV以上的邻苯二酚的氧化电位进行粗调和微调,这是控制深共熔溶剂中质子耦合电子转移的热力学的第一个证明。改变HBD上的取代基可在数十毫伏的范围内进行精细控制,而改变有机盐阴离子的基本强度可在数百毫伏的范围内进行全面控制。
更新日期:2017-11-21
中文翻译:

基于邻苯二酚的深共熔溶剂中的质子耦合电子转移和取代基效应:氧化还原活性的总体和精细调整
各种取代的邻苯二酚中的1,2-二醇部分允许与氯化胆碱或柠檬酸胆碱二氢盐以2:1的比例形成室温离子熔体。这些深度共晶溶剂在氧化还原活性邻苯二酚中为4.3–6.6M。3-和4-取代的邻苯二酚上的取代基使E °和p K a都发生位移,以使Hammett参数预测在方波伏安法中观察到的E p对氧化的作用。用于质子偶联氧化的质子受体比在此报道的取代基和受体中的取代基更强烈地移动观察到的E p。通过p K a可以很好地预测该变化本研究中质子受体的共轭酸,即水溶液中的水或DES中的氯化物或柠檬酸二氢盐的含量。取代基和质子受体共同作用,可对750 mV以上的邻苯二酚的氧化电位进行粗调和微调,这是控制深共熔溶剂中质子耦合电子转移的热力学的第一个证明。改变HBD上的取代基可在数十毫伏的范围内进行精细控制,而改变有机盐阴离子的基本强度可在数百毫伏的范围内进行全面控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号