当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of steam and CO2 on ethane activation over Zn-ZSM-5†
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1039/c7cy01850a Ali Mehdad 1, 2, 3, 4, 5 , Nicholas S. Gould 1, 2, 3, 4, 5 , Bingjun Xu 1, 2, 3, 4, 5 , Raul F. Lobo 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1039/c7cy01850a Ali Mehdad 1, 2, 3, 4, 5 , Nicholas S. Gould 1, 2, 3, 4, 5 , Bingjun Xu 1, 2, 3, 4, 5 , Raul F. Lobo 1, 2, 3, 4, 5
Affiliation
|
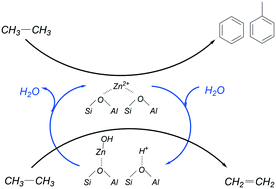
The impact of steam and CO2 on ethane activation over 5% (w/w) Zn-ZSM-5 catalysts was investigated. The main products in He, steam and CO2 were mixtures of ethene, methane, benzene and toluene. CO2 clearly increased the formation rate of ethene and CO, and steam increased the formation rate of ethene. In addition, the presence of CO2 decreased the formation rates of methane and aromatics by factors of ∼3 and 7, respectively. Hydrogen formed from ethane dehydrogenation reacted with CO2, via the reverse water gas shift reaction, to form CO and water. Under vacuum, FTIR spectra of adsorbed ethene on Zn-ZSM-5 showed stronger adsorption on zinc Lewis acid sites than on Brønsted acid sites. In the presence of steam, strong adsorption of ethene on the zinc Lewis acid sites (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C at 1595 cm−1) splits the spectra into two absorption bands (at 1650 and 1568 cm−1), implying the hydrolysis of Zn(II) sites to form Brønsted acid sites and Zn(OH)+ sites. Hydrolysis of Zn(II) sites suppresses oligomerization/aromatization reactions. The changes in selectivity are reversible and can be stopped by decreasing water vapor pressure. The results confirm that Zn(OH)+ sites are effective in ethane dehydrogenation, but the Zn(II) sites are necessary for aromatization. The absence of aromatization reactions in the presence of steam shows that Zn(II) sites catalyze aromatization at faster rates than Brønsted acid sites.
C at 1595 cm−1) splits the spectra into two absorption bands (at 1650 and 1568 cm−1), implying the hydrolysis of Zn(II) sites to form Brønsted acid sites and Zn(OH)+ sites. Hydrolysis of Zn(II) sites suppresses oligomerization/aromatization reactions. The changes in selectivity are reversible and can be stopped by decreasing water vapor pressure. The results confirm that Zn(OH)+ sites are effective in ethane dehydrogenation, but the Zn(II) sites are necessary for aromatization. The absence of aromatization reactions in the presence of steam shows that Zn(II) sites catalyze aromatization at faster rates than Brønsted acid sites.
中文翻译:

蒸汽和CO 2对Zn-ZSM-5上乙烷活化的影响†
研究了蒸汽和CO 2对5%(w / w)Zn-ZSM-5催化剂上乙烷活化的影响。He,蒸汽和CO 2中的主要产物是乙烯,甲烷,苯和甲苯的混合物。CO 2明显增加了乙烯和CO的形成速率,而蒸汽增加了乙烯的形成速率。此外,CO 2的存在将甲烷和芳烃的形成速率分别降低了约3和7倍。从乙烷脱氢形成的氢与CO反应2,经由逆水煤气变换反应,生成一氧化碳和水。在真空下,Zn-ZSM-5上吸附的乙烯的FTIR光谱显示在路易斯酸锌上的吸附力强于布朗斯台德酸上的吸附力。在蒸汽,乙烯的强吸附在锌的路易斯酸位点(存在ν C ^![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) C ^在1595厘米-1)拆分成光谱两个吸收带(在1650和1568厘米-1),这意味着Zn中的水解(II)位点形成布朗斯台德酸位点和Zn(OH)+位点。Zn(II)的水解)位点抑制低聚/芳香化反应。选择性的变化是可逆的,可以通过降低水蒸气压力来停止。结果证实Zn(OH)+位点在乙烷脱氢中是有效的,但是Zn(II)位点对于芳构化是必需的。在蒸汽存在下不存在芳构化反应,这表明Zn(II)部位催化芳构化的速率比布朗斯台德酸部位更快。
C ^在1595厘米-1)拆分成光谱两个吸收带(在1650和1568厘米-1),这意味着Zn中的水解(II)位点形成布朗斯台德酸位点和Zn(OH)+位点。Zn(II)的水解)位点抑制低聚/芳香化反应。选择性的变化是可逆的,可以通过降低水蒸气压力来停止。结果证实Zn(OH)+位点在乙烷脱氢中是有效的,但是Zn(II)位点对于芳构化是必需的。在蒸汽存在下不存在芳构化反应,这表明Zn(II)部位催化芳构化的速率比布朗斯台德酸部位更快。
更新日期:2017-11-20
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C at 1595 cm−1) splits the spectra into two absorption bands (at 1650 and 1568 cm−1), implying the hydrolysis of Zn(II) sites to form Brønsted acid sites and Zn(OH)+ sites. Hydrolysis of Zn(II) sites suppresses oligomerization/aromatization reactions. The changes in selectivity are reversible and can be stopped by decreasing water vapor pressure. The results confirm that Zn(OH)+ sites are effective in ethane dehydrogenation, but the Zn(II) sites are necessary for aromatization. The absence of aromatization reactions in the presence of steam shows that Zn(II) sites catalyze aromatization at faster rates than Brønsted acid sites.
C at 1595 cm−1) splits the spectra into two absorption bands (at 1650 and 1568 cm−1), implying the hydrolysis of Zn(II) sites to form Brønsted acid sites and Zn(OH)+ sites. Hydrolysis of Zn(II) sites suppresses oligomerization/aromatization reactions. The changes in selectivity are reversible and can be stopped by decreasing water vapor pressure. The results confirm that Zn(OH)+ sites are effective in ethane dehydrogenation, but the Zn(II) sites are necessary for aromatization. The absence of aromatization reactions in the presence of steam shows that Zn(II) sites catalyze aromatization at faster rates than Brønsted acid sites.
中文翻译:

蒸汽和CO 2对Zn-ZSM-5上乙烷活化的影响†
研究了蒸汽和CO 2对5%(w / w)Zn-ZSM-5催化剂上乙烷活化的影响。He,蒸汽和CO 2中的主要产物是乙烯,甲烷,苯和甲苯的混合物。CO 2明显增加了乙烯和CO的形成速率,而蒸汽增加了乙烯的形成速率。此外,CO 2的存在将甲烷和芳烃的形成速率分别降低了约3和7倍。从乙烷脱氢形成的氢与CO反应2,经由逆水煤气变换反应,生成一氧化碳和水。在真空下,Zn-ZSM-5上吸附的乙烯的FTIR光谱显示在路易斯酸锌上的吸附力强于布朗斯台德酸上的吸附力。在蒸汽,乙烯的强吸附在锌的路易斯酸位点(存在ν C ^
![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) C ^在1595厘米-1)拆分成光谱两个吸收带(在1650和1568厘米-1),这意味着Zn中的水解(II)位点形成布朗斯台德酸位点和Zn(OH)+位点。Zn(II)的水解)位点抑制低聚/芳香化反应。选择性的变化是可逆的,可以通过降低水蒸气压力来停止。结果证实Zn(OH)+位点在乙烷脱氢中是有效的,但是Zn(II)位点对于芳构化是必需的。在蒸汽存在下不存在芳构化反应,这表明Zn(II)部位催化芳构化的速率比布朗斯台德酸部位更快。
C ^在1595厘米-1)拆分成光谱两个吸收带(在1650和1568厘米-1),这意味着Zn中的水解(II)位点形成布朗斯台德酸位点和Zn(OH)+位点。Zn(II)的水解)位点抑制低聚/芳香化反应。选择性的变化是可逆的,可以通过降低水蒸气压力来停止。结果证实Zn(OH)+位点在乙烷脱氢中是有效的,但是Zn(II)位点对于芳构化是必需的。在蒸汽存在下不存在芳构化反应,这表明Zn(II)部位催化芳构化的速率比布朗斯台德酸部位更快。











































 京公网安备 11010802027423号
京公网安备 11010802027423号