当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The cooperation effect in the Au–Pd/LDH for promoting photocatalytic selective oxidation of benzyl alcohol†
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1039/c7cy02006f Zhitong Wang 1, 2, 3, 4 , Yujie Song 1, 2, 3, 4 , Junhua Zou 1, 2, 3, 4 , Liuyi Li 2, 3, 4, 5 , Yan Yu 2, 3, 4, 5 , Ling Wu 1, 2, 3, 4
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1039/c7cy02006f Zhitong Wang 1, 2, 3, 4 , Yujie Song 1, 2, 3, 4 , Junhua Zou 1, 2, 3, 4 , Liuyi Li 2, 3, 4, 5 , Yan Yu 2, 3, 4, 5 , Ling Wu 1, 2, 3, 4
Affiliation
|
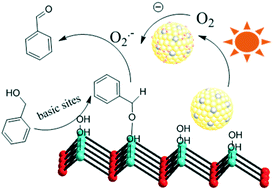
Materials with different Au–Pd ratios bimetallic alloy nanoparticles (NPs) supported on MgAl–LDH (Au–Pd/LDH) were prepared by an impregnation–reduction method and developed as photocatalysts for the selective oxidation of benzyl alcohol to benzaldehyde under visible light irradiation. A typical sample (Au9–Pd1/LDH) showed efficient photocatalytic activity with 91.1% conversion and 99% selectivity. The conversion was greatly increased from 3.6% for Au/LDH and 1.5% for Pd/LDH, respectively. Moreover, it was four times higher than that for Au9–Pd1/TiO2. The ESR spectrum demonstrated that the main reactive species were superoxide radicals (O2˙−) generated by the transfer of hot electrons from the Au–Pd alloy NPs to the surface adsorbed molecular oxygen. The formation of hot electrons was attributed to localized surface plasmon resonance (LSPR) of the alloy NPs. Based on XPS analysis, a synergistic electron effect in the Au–Pd alloy was observed due to the transfer of electrons from Pd to Au atoms. This effect enhances the adsorption ability of Pd atoms to oxygen molecules and facilitates the formation of O2˙−. The results from CO2-TPD and in situ FT-IR analyses indicated that benzyl alcohol could be efficiently activated at the surface base sites on the LDH via surface coordination to form a metal-alkoxide intermediate. Here, the intermediate could be more easily oxidized by O2˙− to form benzaldehyde. Finally, a possible mechanism based on the cooperation effect between the alloy NPs and the surface basic sites on the LDH was proposed to illustrate the photocatalytic process.
中文翻译:

Au-Pd / LDH中的协同作用促进苄醇的光催化选择性氧化†
通过浸渍-还原法制备负载在MgAl-LDH(Au-Pd / LDH)上的具有不同Au-Pd比的双金属合金纳米颗粒(NPs)的材料,并开发了用作可见光照射下苯甲醇选择性氧化为苯甲醛的光催化剂。 。典型样品(Au 9 -Pd 1 / LDH)显示出有效的光催化活性,转化率为91.1%,选择性为99%。转化率分别从Au / LDH的3.6%和Pd / LDH的1.5%大大提高。而且,它是Au 9 -Pd 1 / TiO 2的四倍。ESR谱表明,主要的反应性物质是超氧自由基(O 2 ˙ -)是由于热电子从Au-Pd合金NPs转移到表面吸附的分子氧而产生的。热电子的形成归因于合金NP的局部表面等离振子共振(LSPR)。根据XPS分析,由于电子从Pd转移到Au原子,因此在Au-Pd合金中观察到了协同电子效应。这种效果增强了钯原子的氧分子吸附能力和促进的O-形成2 ˙ - 。从CO结果2 -TPD和原位FT-IR分析表明,苯甲醇可以在对LDH表面碱位被有效地激活经由表面配位形成金属醇盐中间体。在此,中间可以更容易地被O氧化2 ˙ -以形成苯甲醛。最后,基于合金NP与LDH表面碱性位点之间的协同作用,提出了一种可能的机理来说明光催化过程。
更新日期:2017-11-20
中文翻译:

Au-Pd / LDH中的协同作用促进苄醇的光催化选择性氧化†
通过浸渍-还原法制备负载在MgAl-LDH(Au-Pd / LDH)上的具有不同Au-Pd比的双金属合金纳米颗粒(NPs)的材料,并开发了用作可见光照射下苯甲醇选择性氧化为苯甲醛的光催化剂。 。典型样品(Au 9 -Pd 1 / LDH)显示出有效的光催化活性,转化率为91.1%,选择性为99%。转化率分别从Au / LDH的3.6%和Pd / LDH的1.5%大大提高。而且,它是Au 9 -Pd 1 / TiO 2的四倍。ESR谱表明,主要的反应性物质是超氧自由基(O 2 ˙ -)是由于热电子从Au-Pd合金NPs转移到表面吸附的分子氧而产生的。热电子的形成归因于合金NP的局部表面等离振子共振(LSPR)。根据XPS分析,由于电子从Pd转移到Au原子,因此在Au-Pd合金中观察到了协同电子效应。这种效果增强了钯原子的氧分子吸附能力和促进的O-形成2 ˙ - 。从CO结果2 -TPD和原位FT-IR分析表明,苯甲醇可以在对LDH表面碱位被有效地激活经由表面配位形成金属醇盐中间体。在此,中间可以更容易地被O氧化2 ˙ -以形成苯甲醛。最后,基于合金NP与LDH表面碱性位点之间的协同作用,提出了一种可能的机理来说明光催化过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号