Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stable isotope labeling by essential nutrients in cell culture (SILEC) for accurate measurement of nicotinamide adenine dinucleotide metabolism
Analyst ( IF 3.6 ) Pub Date : 2017-10-18 00:00:00 , DOI: 10.1039/c7an01378g David W. Frederick 1, 2, 3, 4, 5 , Sophie Trefely 6, 7, 8, 9 , Alexia Buas 6, 7, 8, 9 , Jason Goodspeed 6, 7, 8, 9 , Jay Singh 6, 7, 8, 9 , Clementina Mesaros 5, 8, 9, 10, 11 , Joseph A. Baur 1, 2, 3, 4, 5 , Nathaniel W. Snyder 6, 7, 8, 9
Analyst ( IF 3.6 ) Pub Date : 2017-10-18 00:00:00 , DOI: 10.1039/c7an01378g David W. Frederick 1, 2, 3, 4, 5 , Sophie Trefely 6, 7, 8, 9 , Alexia Buas 6, 7, 8, 9 , Jason Goodspeed 6, 7, 8, 9 , Jay Singh 6, 7, 8, 9 , Clementina Mesaros 5, 8, 9, 10, 11 , Joseph A. Baur 1, 2, 3, 4, 5 , Nathaniel W. Snyder 6, 7, 8, 9
Affiliation
|
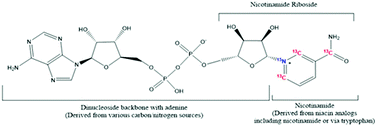
Nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP) are conserved metabolic cofactors that mediate reduction-oxidation (redox) reactions throughout all domains of life. The diversity of synthetic routes and cellular processes involving the transfer of reducing equivalents to and from these cofactors makes the accurate quantitation and metabolic tracing of NAD(H) and NADP(H) of broad interest. However, current analytical techniques typically rely on standard curves that do not incorporate confounding effects of the sample matrix. We utilized the essential requirement of niacin and tryptophan for NAD synthesis in mammalian cells to devise a stable isotope labeling by essential nutrients in cell culture (SILEC) method for efficient labeling of intracellular NAD(H) and NADP(H) pools. Coupling this approach with detection by liquid chromatography-high resolution mass spectrometry (LC-HRMS), we demonstrate the utility of incorporating a [13C315N1]-nicotinamide moiety into a library of NAD-derived metabolites for use as internal standards in matrixed samples. Using a two-label system incorporating [13C315N1]-nicotinamide and [13C11]-tryptophan, we quantify the relative contribution of salvage and de novo NAD synthesis, respectively, in S. cerevisiae and HepG2 human hepatocellular carcinoma cells under basal conditions. As a further proof-of-principle, we demonstrate an improvement in the linear range for quantification of NAD and apply this method to analysis of NAD(H) in mouse liver. This method demonstrates the generalizability of SILEC, and provides a simple method for generating a library of stable isotope labeled internal standards for quantifying and tracing the metabolism of cellular and tissue NAD(H) and NADP(H).
中文翻译:

通过细胞培养中的基本营养素(SILEC)进行稳定的同位素标记,可准确测量烟酰胺腺嘌呤二核苷酸代谢
烟酰胺腺嘌呤二核苷酸(NAD)和烟酰胺腺嘌呤二核苷酸磷酸(NADP)是保守的代谢辅因子,可在生活的所有领域中介导还原-氧化(redox)反应。合成途径和细胞过程的多样性,涉及到与这些辅因子之间的还原等效物的转移,使得对NAD(H)和NADP(H)的准确定量和代谢追踪成为人们广泛关注的问题。但是,当前的分析技术通常依赖于标准曲线,该标准曲线不包含样品基质的混杂效应。我们利用烟酸和色氨酸对哺乳动物细胞中NAD合成的基本要求,通过细胞培养(SILEC)方法中的基本营养素来设计稳定的同位素标记,以有效标记细胞内NAD(H)和NADP(H)库。将13 C 3 15 N 1 ]-烟酰胺部分放入NAD衍生的代谢物文库中,用作基质样品中的内标。使用结合了[ 13 C 3 15 N 1 ]-烟酰胺和[ 13 C 11 ]-色氨酸的双标签系统,我们分别定量了酿酒酵母中打捞和从头NAD合成的相对贡献。基础条件下的HepG2和HepG2人肝癌细胞。作为进一步的原理证明,我们证明了线性范围内对NAD定量的改进,并将该方法应用于小鼠肝脏中NAD(H)的分析。此方法证明了SILEC的通用性,并提供了一种简单的方法来生成稳定同位素标记的内标库,以定量和跟踪细胞和组织NAD(H)和NADP(H)的代谢。
更新日期:2017-11-20
中文翻译:

通过细胞培养中的基本营养素(SILEC)进行稳定的同位素标记,可准确测量烟酰胺腺嘌呤二核苷酸代谢
烟酰胺腺嘌呤二核苷酸(NAD)和烟酰胺腺嘌呤二核苷酸磷酸(NADP)是保守的代谢辅因子,可在生活的所有领域中介导还原-氧化(redox)反应。合成途径和细胞过程的多样性,涉及到与这些辅因子之间的还原等效物的转移,使得对NAD(H)和NADP(H)的准确定量和代谢追踪成为人们广泛关注的问题。但是,当前的分析技术通常依赖于标准曲线,该标准曲线不包含样品基质的混杂效应。我们利用烟酸和色氨酸对哺乳动物细胞中NAD合成的基本要求,通过细胞培养(SILEC)方法中的基本营养素来设计稳定的同位素标记,以有效标记细胞内NAD(H)和NADP(H)库。将13 C 3 15 N 1 ]-烟酰胺部分放入NAD衍生的代谢物文库中,用作基质样品中的内标。使用结合了[ 13 C 3 15 N 1 ]-烟酰胺和[ 13 C 11 ]-色氨酸的双标签系统,我们分别定量了酿酒酵母中打捞和从头NAD合成的相对贡献。基础条件下的HepG2和HepG2人肝癌细胞。作为进一步的原理证明,我们证明了线性范围内对NAD定量的改进,并将该方法应用于小鼠肝脏中NAD(H)的分析。此方法证明了SILEC的通用性,并提供了一种简单的方法来生成稳定同位素标记的内标库,以定量和跟踪细胞和组织NAD(H)和NADP(H)的代谢。











































 京公网安备 11010802027423号
京公网安备 11010802027423号