Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comprehensive Understanding of the Kinetics and Mechanism of Fluoride Removal over a Potent Nanocrystalline Hydroxyapatite Surface
ACS Omega ( IF 3.7 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1021/acsomega.7b00370 Bishnupriya Nayak 1 , Amruta Samant 1 , Rajkishore Patel 2 , Pramila K. Misra 1
ACS Omega ( IF 3.7 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1021/acsomega.7b00370 Bishnupriya Nayak 1 , Amruta Samant 1 , Rajkishore Patel 2 , Pramila K. Misra 1
Affiliation
|
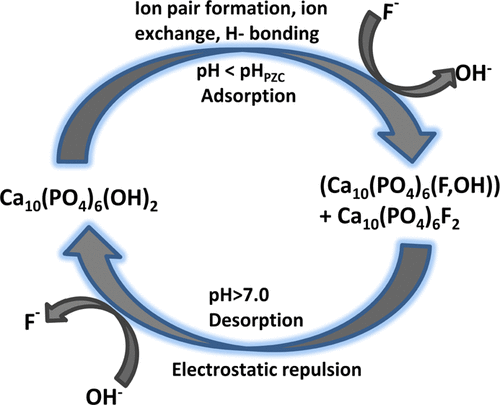
Hydroxyapatite (HAp) was successfully synthesized from egg shells, a low cost and easily available biodegradable waste, by the precipitation method and characterized by X-ray diffraction (XRD), scanning electron microscopy, Fourier transform infrared, and Brunauer–Emmett–Teller (BET) surface area analysis. The surface area of HAp was found to be 144 m2/g with a crystalline size of 9–99 nm from the BET and XRD data. The maximum fluoride removal efficiency within 1 h using 0.3 g of the synthesized adsorbent at pH 6 was 95%. The adsorption of fluoride followed second-order kinetics, indicating that chemisorptions are the rate-limiting step. The experimental data were well fitted with Langmuir and Freundlich isotherms, validating both monolayer and multilayer sorption during the fluoride adsorption onto the porous HAp. The positive adsorption of F– ions at the HAp interface can be attributed to ion exchange/ion pairing and H-bonding below the pHpzc of HAp (pHpzc = 8), and the negative adsorption can be attributed to the electrostatic repulsion between O– and F– ions at alkaline pH. Both physical and chemical adsorption phenomena were also evidenced from the molecular parking area data. The results of a batch experiment show that the HAp synthesized from egg shells can be used as an effective, low-cost adsorbent for fluoride removal from a contaminated aqueous solution as well as groundwater compared to other adsorbents.
中文翻译:

全面了解有效的纳米晶羟基磷灰石表面除氟的动力学和机理
羟基磷灰石(HAp)是通过沉淀法成功地从蛋壳中合成的,该壳是低成本且易于获得的可生物降解废物,并通过X射线衍射(XRD),扫描电子显微镜,傅里叶变换红外光谱和Brunauer-Emmett-Teller( BET)表面积分析。发现HAp的表面积为144 m 2/ g,根据BET和XRD数据,晶体大小为9–99 nm。使用0.3 g的合成吸附剂在pH 6下1 h内的最大除氟效率为95%。氟化物的吸附遵循二级动力学,表明化学吸附是限速步骤。实验数据与Langmuir和Freundlich等温线非常吻合,验证了氟化物吸附到多孔HAp上的单层和多层吸附。F的正吸附-离子在羟基磷灰石接口可以归因于离子交换/离子配对和H-键合的pH值低于PZC的HAp液(pH PZC = 8),和负的吸附可以归因于-O之间的静电排斥–和F –碱性pH下的离子。分子停车区数据也证明了物理和化学吸附现象。批处理实验的结果表明,与其他吸附剂相比,从蛋壳合成的HAp可作为一种有效,低成本的吸附剂,用于从受污染的水溶液和地下水中去除氟化物。
更新日期:2017-11-20
中文翻译:

全面了解有效的纳米晶羟基磷灰石表面除氟的动力学和机理
羟基磷灰石(HAp)是通过沉淀法成功地从蛋壳中合成的,该壳是低成本且易于获得的可生物降解废物,并通过X射线衍射(XRD),扫描电子显微镜,傅里叶变换红外光谱和Brunauer-Emmett-Teller( BET)表面积分析。发现HAp的表面积为144 m 2/ g,根据BET和XRD数据,晶体大小为9–99 nm。使用0.3 g的合成吸附剂在pH 6下1 h内的最大除氟效率为95%。氟化物的吸附遵循二级动力学,表明化学吸附是限速步骤。实验数据与Langmuir和Freundlich等温线非常吻合,验证了氟化物吸附到多孔HAp上的单层和多层吸附。F的正吸附-离子在羟基磷灰石接口可以归因于离子交换/离子配对和H-键合的pH值低于PZC的HAp液(pH PZC = 8),和负的吸附可以归因于-O之间的静电排斥–和F –碱性pH下的离子。分子停车区数据也证明了物理和化学吸附现象。批处理实验的结果表明,与其他吸附剂相比,从蛋壳合成的HAp可作为一种有效,低成本的吸附剂,用于从受污染的水溶液和地下水中去除氟化物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号