当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Melting Behavior and Solubility Equilibria of (R)- and (S)-Mefenpyr-diethyl in Ethanol/Water Mixtures
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1021/acs.jced.7b00819 Anne-K. Kort 1 , Heike Lorenz 1 , Andreas Seidel-Morgenstern 1, 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1021/acs.jced.7b00819 Anne-K. Kort 1 , Heike Lorenz 1 , Andreas Seidel-Morgenstern 1, 2
Affiliation
|
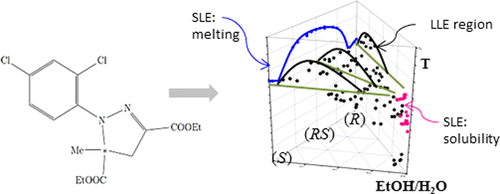
Thermodynamic dependencies and information are of prime importance in performing a controlled selective crystallization process to separate, e.g., the enantiomers of chiral compounds. This study is focused on the determination of phase diagrams of a chiral system. The melting behavior and solubility equilibria (SLE) of diethyl-1-(2,4-dichlorophenyl)-5-methyl-4,5-dihydro-1H-pyrazole-3,5-dicarboxylate (mefenpyr-diethyl) in various ethanol/water mixtures in the temperature range of (273 to 293) K were analyzed. The obtained results identify mefenpyr-diethyl as a racemic compound-forming system. It has been found that the solubility behavior strongly depends on temperature. In certain concentration ranges, liquid–liquid equilibria (LLE) were detected. Liquid–liquid separation has to be prevented during cooling crystallization processes. The observed LLE phenomena were quantified within this study in polythermal (turbidity) measurements, and the phase diagrams were derived on the basis of the experimental data. With these diagrams, a successful selective crystallization process avoiding oiling out is feasible.
中文翻译:

(R)-和(S)-Mefenpyr-二乙基在乙醇/水混合物中的熔融行为和溶解度平衡
在进行受控的选择性结晶过程以分离例如手性化合物的对映异构体时,热力学依赖性和信息至关重要。这项研究的重点是确定手性系统的相图。乙基-1-(2,4-二氯苯基)-5-甲基-4,5-二氢-1 H的熔融行为和溶解度平衡(SLE)分析了在(273至293)K温度范围内的各种乙醇/水混合物中的-吡唑-3,5-二羧酸酯(甲芬比-二乙基)。所得结果确定了甲芬比尔-二乙基为外消旋化合物形成系统。已经发现,溶解度行为强烈地取决于温度。在某些浓度范围内,检测到液-液平衡(LLE)。在冷却结晶过程中必须防止液-液分离。在这项研究中,通过多热(浊度)测量对观察到的LLE现象进行了量化,并且根据实验数据得出了相图。使用这些图,成功的选择性结晶过程避免了油蚀是可行的。
更新日期:2017-11-20
中文翻译:

(R)-和(S)-Mefenpyr-二乙基在乙醇/水混合物中的熔融行为和溶解度平衡
在进行受控的选择性结晶过程以分离例如手性化合物的对映异构体时,热力学依赖性和信息至关重要。这项研究的重点是确定手性系统的相图。乙基-1-(2,4-二氯苯基)-5-甲基-4,5-二氢-1 H的熔融行为和溶解度平衡(SLE)分析了在(273至293)K温度范围内的各种乙醇/水混合物中的-吡唑-3,5-二羧酸酯(甲芬比-二乙基)。所得结果确定了甲芬比尔-二乙基为外消旋化合物形成系统。已经发现,溶解度行为强烈地取决于温度。在某些浓度范围内,检测到液-液平衡(LLE)。在冷却结晶过程中必须防止液-液分离。在这项研究中,通过多热(浊度)测量对观察到的LLE现象进行了量化,并且根据实验数据得出了相图。使用这些图,成功的选择性结晶过程避免了油蚀是可行的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号