当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pure and Binary Gas Adsorption Equilibrium for CO2–N2 on Oxygen Enriched Nanostructured Carbon Adsorbents
Energy & Fuels ( IF 5.2 ) Pub Date : 2017-12-05 00:00:00 , DOI: 10.1021/acs.energyfuels.7b02671 Chitrakshi Goel 1 , Deepak Tiwari 1 , Haripada Bhunia 1 , Pramod K. Bajpai 1
Energy & Fuels ( IF 5.2 ) Pub Date : 2017-12-05 00:00:00 , DOI: 10.1021/acs.energyfuels.7b02671 Chitrakshi Goel 1 , Deepak Tiwari 1 , Haripada Bhunia 1 , Pramod K. Bajpai 1
Affiliation
|
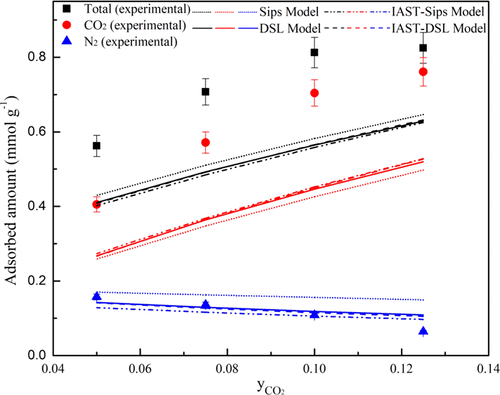
Pure component (CO2 and N2) adsorption isotherms of oxygen enriched nanostructured carbon (RF-700) were evaluated using a static volumetric analyzer at four different adsorption temperatures ranging from 30 to 100 °C. Langmuir, Sips, and dual-site Langmuir (DSL) models were used to correlate pure component adsorption isotherms and it was found that Sips and DSL isotherm model fitted well with the experimental data, indicating the heterogeneous nature of the adsorbent surface. Fixed-bed column was used to obtain dynamic breakthrough data for binary system CO2–N2 at different adsorption temperatures (30–100 °C) and CO2 feed concentrations (5–12.5% by volume). Extended Sips, extended DSL, and IAST (ideal adsorbed solution theory) models using pure component adsorption isotherm data were used for the prediction of adsorption of binary system (CO2–N2). Predicted equilibria data was compared with experimental breakthrough curve data, and it was found that extended forms of the isotherm models (Sips and DSL) underpredicted CO2 adsorption equilibria because of the difference in adsorptive strengths of CO2 and N2 molecules. Ideal adsorbed solution theory failed to describe the mixed-gas adsorption equilibria. Asymmetric x–y diagrams showed positive deviation from Raoult’s law. The feasibility of the adsorption process was suggested by the negative value of molar Gibbs free energy change. The formation of more ordered configuration of CO2 molecules on the adsorbent surface was seen as a higher heat of adsorption was exhibited for CO2 as compared to N2.
中文翻译:

富氧纳米结构碳吸附剂对CO 2 -N 2的纯和二元气体吸附平衡
使用静态体积分析仪在30至100°C的四种不同吸附温度下评估了富氧纳米结构碳(RF-700)的纯组分(CO 2和N 2)吸附等温线。用Langmuir,Sips和双站点Langmuir(DSL)模型关联纯组分吸附等温线,发现Sips和DSL等温线模型与实验数据吻合得很好,表明了吸附剂表面的非均质性。使用固定床色谱柱获得二元系统CO 2 –N 2在不同吸附温度(30–100°C)和CO 2下的动态突破数据饲料浓度(5-12.5%(体积))。使用纯组分吸附等温线数据的扩展Sips,扩展DSL和IAST(理想吸附溶液理论)模型用于预测二元系统(CO 2 -N 2)的吸附。将预测的平衡数据与实验突破曲线数据进行了比较,发现等温线模型的扩展形式(Sips和DSL)由于CO 2和N 2分子的吸附强度不同而导致预测的CO 2吸附平衡不足。理想的吸附溶液理论无法描述混合气体的吸附平衡。不对称x–y图表显示出与拉乌尔定律的正偏差。摩尔吉布斯自由能变化的负值表明了吸附过程的可行性。可以看出,在吸附剂表面上形成了更有序的CO 2分子构型,因为与N 2相比,CO 2表现出更高的吸附热。
更新日期:2017-12-05
中文翻译:

富氧纳米结构碳吸附剂对CO 2 -N 2的纯和二元气体吸附平衡
使用静态体积分析仪在30至100°C的四种不同吸附温度下评估了富氧纳米结构碳(RF-700)的纯组分(CO 2和N 2)吸附等温线。用Langmuir,Sips和双站点Langmuir(DSL)模型关联纯组分吸附等温线,发现Sips和DSL等温线模型与实验数据吻合得很好,表明了吸附剂表面的非均质性。使用固定床色谱柱获得二元系统CO 2 –N 2在不同吸附温度(30–100°C)和CO 2下的动态突破数据饲料浓度(5-12.5%(体积))。使用纯组分吸附等温线数据的扩展Sips,扩展DSL和IAST(理想吸附溶液理论)模型用于预测二元系统(CO 2 -N 2)的吸附。将预测的平衡数据与实验突破曲线数据进行了比较,发现等温线模型的扩展形式(Sips和DSL)由于CO 2和N 2分子的吸附强度不同而导致预测的CO 2吸附平衡不足。理想的吸附溶液理论无法描述混合气体的吸附平衡。不对称x–y图表显示出与拉乌尔定律的正偏差。摩尔吉布斯自由能变化的负值表明了吸附过程的可行性。可以看出,在吸附剂表面上形成了更有序的CO 2分子构型,因为与N 2相比,CO 2表现出更高的吸附热。











































 京公网安备 11010802027423号
京公网安备 11010802027423号