当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental Measurement of CO2 Solubility in Aqueous CaCl2 Solution at Temperature from 323.15 to 423.15 K and Pressure up to 20 MPa Using the Conductometric Titration
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.jced.7b00591 Hamdi Messabeb 1 , François Contamine 1 , Pierre Cézac 1 , Jean Paul Serin 1 , Clémentine Pouget 1 , Eric C. Gaucher 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.jced.7b00591 Hamdi Messabeb 1 , François Contamine 1 , Pierre Cézac 1 , Jean Paul Serin 1 , Clémentine Pouget 1 , Eric C. Gaucher 2
Affiliation
|
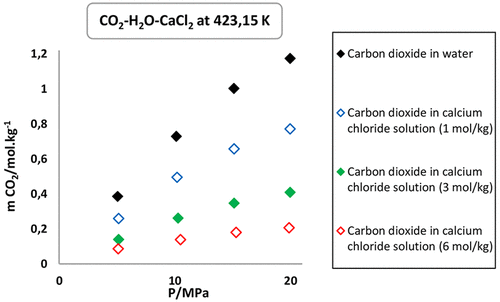
In the framework of the efforts of the scientific community developed for the reduction of CO2 emissions, the geological storage of CO2 in deep saline aquifers is under focus. An increase of salinity decreases the potential of CO2 solubilization into the water. In salty waters, the salinity is not only due to NaCl but also to others ions and in particular Ca and Mg. Experimental solubility data of CO2 in calcium chloride solution available in the literature at conditions relevant to carbon storage are particularly scarce. In this work, a new analytical method was developed for experimental measurement of CO2 solubility in calcium chloride solutions (1, 3, and 6 mol/kg) at high pressures (5–20 MPa) and temperatures (323.15, 373.15, and 423.15 K). This method is based on conductometric titration coupled with classical pH titration. The conductimetry shows sharper curves than the pH titration allowing a higher precision. Thirty-six new experimental data are reported in this paper. These data presented an experimental average uncertainty of 2.1% with the ANOVA calculation method based on repeatability and reproducibility experiments. The CO2 solubility in CaCl2 solutions is noticeable lower than in NaCl solution increasing the salting out effect. Considering our previous work on NaCl solutions and this paper for CaCl2 solutions, estimations of the real quantity of CO2 that may be dissolved in saline aquifers can be made with a significantly better precision.
中文翻译:

电导滴定法在温度为323.15至423.15 K,压力高达20 MPa的条件下测量CaCl 2水溶液中CO 2溶解度的实验
在科学界为减少CO 2排放量而做出的努力的框架内,深层盐水层中CO 2的地质储藏成为人们关注的重点。盐度的增加降低了将CO 2溶解到水中的可能性。在咸水中,盐度不仅归因于NaCl,还归因于其他离子,尤其是Ca和Mg。文献中在与碳储存有关的条件下可获得的实验结果表明,CO 2在氯化钙溶液中的溶解度数据很少。在这项工作中,为实验测量CO 2开发了一种新的分析方法。在高压(5-20 MPa)和温度(323.15、373.15和423.15 K)下在氯化钙溶液(1、3和6 mol / kg)中的溶解度。该方法基于电导滴定和经典pH滴定。电导率曲线显示出比pH滴定曲线更锐利的曲线,可实现更高的精度。本文报道了三十六项新的实验数据。这些数据基于可重复性和可再现性实验的ANOVA计算方法得出的实验平均不确定度为2.1%。在CaCl 2溶液中的CO 2溶解度明显低于在NaCl溶液中的溶解度,从而增加了盐析作用。考虑到我们之前在NaCl溶液方面的工作以及本文针对CaCl 2的研究可以用明显更好的精度估算可能溶解在盐水层中的CO 2的实际量。
更新日期:2017-11-19
中文翻译:

电导滴定法在温度为323.15至423.15 K,压力高达20 MPa的条件下测量CaCl 2水溶液中CO 2溶解度的实验
在科学界为减少CO 2排放量而做出的努力的框架内,深层盐水层中CO 2的地质储藏成为人们关注的重点。盐度的增加降低了将CO 2溶解到水中的可能性。在咸水中,盐度不仅归因于NaCl,还归因于其他离子,尤其是Ca和Mg。文献中在与碳储存有关的条件下可获得的实验结果表明,CO 2在氯化钙溶液中的溶解度数据很少。在这项工作中,为实验测量CO 2开发了一种新的分析方法。在高压(5-20 MPa)和温度(323.15、373.15和423.15 K)下在氯化钙溶液(1、3和6 mol / kg)中的溶解度。该方法基于电导滴定和经典pH滴定。电导率曲线显示出比pH滴定曲线更锐利的曲线,可实现更高的精度。本文报道了三十六项新的实验数据。这些数据基于可重复性和可再现性实验的ANOVA计算方法得出的实验平均不确定度为2.1%。在CaCl 2溶液中的CO 2溶解度明显低于在NaCl溶液中的溶解度,从而增加了盐析作用。考虑到我们之前在NaCl溶液方面的工作以及本文针对CaCl 2的研究可以用明显更好的精度估算可能溶解在盐水层中的CO 2的实际量。










































 京公网安备 11010802027423号
京公网安备 11010802027423号