当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Vapor–Liquid Equilibrium Data for Binary Mixtures of Dimethyl Carbonate with Methyl Acetate, Ethyl Acetate, n-Propyl Acetate, Isopropyl Acetate, n-Butyl Acetate, and Isoamyl Acetate at 93.13 kPa
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.jced.7b00704 Nilesh A. Mali 1 , Satyajeet S. Yadav 2 , Pravin D. Ghuge 1 , Sunil. S. Joshi 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.jced.7b00704 Nilesh A. Mali 1 , Satyajeet S. Yadav 2 , Pravin D. Ghuge 1 , Sunil. S. Joshi 1
Affiliation
|
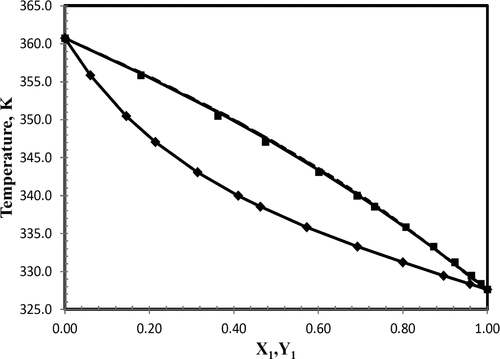
Isobaric vapor–liquid equilibrium (VLE) data was measured at the local atmospheric pressure of 93.13 kPa for the binary systems of dimethyl carbonate (DMC) with methyl acetate, ethyl acetate, n-propyl acetate, isopropyl acetate, n-butyl acetate, and isoamyl acetate using a dynamic recirculation still. VLE data was generated in the form of T–x,y and was checked for thermodynamic consistency using the Herington area test, Van Ness test, and mean absolute deviation between experimental and calculated total pressure and vapor phase composition. Data for all pairs meet the criteria for thermodynamic consistency and were found suitable for process modeling. Binary interaction parameters for the Wilson, nonrandom two-liquid (NRTL), and universal quasichemical (UNIQUAC) activity coefficient models were determined using the objective function of minimizing the deviation between the experimental and the calculated vapor phase composition and total pressure. For all binary systems, Wilson, NRTL, and UNIQUAC models gave good predictions. Azeotropic behavior was observed for the isopropyl acetate–DMC pair at 357.8 K and 0.6 mole fraction of isopropyl acetate.
中文翻译:

碳酸二甲酯与乙酸甲酯,乙酸乙酯,乙酸正丙酯,乙酸异丙酯,乙酸正丁酯和乙酸异戊酯的二元混合物的汽液平衡数据
在碳酸二甲酯(DMC)与乙酸甲酯,乙酸乙酯,乙酸正丙酯,乙酸异丙酯,乙酸正丁酯和乙酸异戊酯仍采用动态再循环。VLE数据以T – x,y的形式生成并使用Herington面积测试,Van Ness测试检查热力学一致性,以及实验和计算的总压力与气相组成之间的平均绝对偏差。所有对的数据均符合热力学一致性的标准,并且发现适合于过程建模。威尔逊,非随机两液(NRTL)和通用拟化学(UNIQUAC)活度系数模型的二元相互作用参数是使用目标函数确定的,该函数将实验和计算的气相组成和总压力之间的偏差最小化。对于所有二进制系统,Wilson,NRTL和UNIQUAC模型都给出了很好的预测。在357.8 K和0.6摩尔分数的乙酸异丙酯上观察到乙酸异丙酯-DMC对的共沸行为。
更新日期:2017-11-19
中文翻译:

碳酸二甲酯与乙酸甲酯,乙酸乙酯,乙酸正丙酯,乙酸异丙酯,乙酸正丁酯和乙酸异戊酯的二元混合物的汽液平衡数据
在碳酸二甲酯(DMC)与乙酸甲酯,乙酸乙酯,乙酸正丙酯,乙酸异丙酯,乙酸正丁酯和乙酸异戊酯仍采用动态再循环。VLE数据以T – x,y的形式生成并使用Herington面积测试,Van Ness测试检查热力学一致性,以及实验和计算的总压力与气相组成之间的平均绝对偏差。所有对的数据均符合热力学一致性的标准,并且发现适合于过程建模。威尔逊,非随机两液(NRTL)和通用拟化学(UNIQUAC)活度系数模型的二元相互作用参数是使用目标函数确定的,该函数将实验和计算的气相组成和总压力之间的偏差最小化。对于所有二进制系统,Wilson,NRTL和UNIQUAC模型都给出了很好的预测。在357.8 K和0.6摩尔分数的乙酸异丙酯上观察到乙酸异丙酯-DMC对的共沸行为。



























 京公网安备 11010802027423号
京公网安备 11010802027423号