Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Extended adjuvant intermittent letrozole versus continuous letrozole in postmenopausal women with breast cancer (SOLE): a multicentre, open-label, randomised, phase 3 trial.
The Lancet ( IF 98.4 ) Pub Date : 2017-11-17 , DOI: 10.1016/s1470-2045(17)30715-5 Marco Colleoni 1 , Weixiu Luo 2 , Per Karlsson 3 , Jacquie Chirgwin 4 , Stefan Aebi 5 , Guy Jerusalem 6 , Patrick Neven 7 , Erika Hitre 8 , Marie-Pascale Graas 9 , Edda Simoncini 10 , Claus Kamby 11 , Alastair Thompson 12 , Sibylle Loibl 13 , Joaquín Gavilá 14 , Katsumasa Kuroi 15 , Christian Marth 16 , Bettina Müller 17 , Seamus O'Reilly 18 , Vincenzo Di Lauro 19 , Andrea Gombos 20 , Thomas Ruhstaller 21 , Harold Burstein 22 , Karin Ribi 23 , Jürg Bernhard 24 , Giuseppe Viale 25 , Rudolf Maibach 23 , Manuela Rabaglio-Poretti 26 , Richard D Gelber 27 , Alan S Coates 28 , Angelo Di Leo 29 , Meredith M Regan 30 , Aron Goldhirsch 31 ,
The Lancet ( IF 98.4 ) Pub Date : 2017-11-17 , DOI: 10.1016/s1470-2045(17)30715-5 Marco Colleoni 1 , Weixiu Luo 2 , Per Karlsson 3 , Jacquie Chirgwin 4 , Stefan Aebi 5 , Guy Jerusalem 6 , Patrick Neven 7 , Erika Hitre 8 , Marie-Pascale Graas 9 , Edda Simoncini 10 , Claus Kamby 11 , Alastair Thompson 12 , Sibylle Loibl 13 , Joaquín Gavilá 14 , Katsumasa Kuroi 15 , Christian Marth 16 , Bettina Müller 17 , Seamus O'Reilly 18 , Vincenzo Di Lauro 19 , Andrea Gombos 20 , Thomas Ruhstaller 21 , Harold Burstein 22 , Karin Ribi 23 , Jürg Bernhard 24 , Giuseppe Viale 25 , Rudolf Maibach 23 , Manuela Rabaglio-Poretti 26 , Richard D Gelber 27 , Alan S Coates 28 , Angelo Di Leo 29 , Meredith M Regan 30 , Aron Goldhirsch 31 ,
Affiliation
|
BACKGROUND
In animal models of breast cancer, resistance to continuous use of letrozole can be reversed by withdrawal and reintroduction of letrozole. We therefore hypothesised that extended intermittent use of adjuvant letrozole would improve breast cancer outcome compared with continuous use of letrozole in postmenopausal women.
METHODS
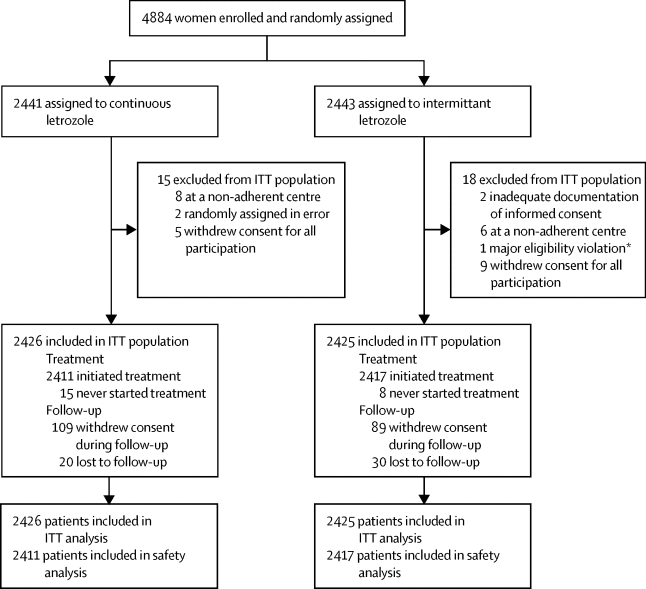
We did the multicentre, open-label, randomised, parallel, phase 3 SOLE trial in 240 centres (academic, primary, secondary, and tertiary care centres) in 22 countries. We enrolled postmenopausal women of any age with hormone receptor-positive, lymph node-positive, and operable breast cancer for which they had undergone local treatment (surgery with or without radiotherapy) and had completed 4-6 years of adjuvant endocrine therapy. They had to be clinically free of breast cancer at enrolment and without evidence of recurrent disease at any time before randomisation. We randomly assigned women (1:1) to treatment groups of either continuous use of letrozole (2·5 mg/day orally for 5 years) or intermittent use of letrozole (2·5 mg/day orally for 9 months followed by a 3-month break in years 1-4 and then 2·5 mg/day during all 12 months of year 5). Randomisation was done by principal investigators or designee at respective centres through the internet-based system of the International Breast Cancer Study Group, was stratified by type of previous endocrine therapy (aromatase inhibitors only vs selective oestrogen receptor modulators only vs both therapies), and used permuted block sizes of four and institutional balancing. No one was masked to treatment assignment. The primary endpoint was disease-free survival, analysed by the intention-to-treat principle using a stratified log-rank test. All patients in the intention-to-treat population who initiated protocol treatment during their period of trial participation were included in the safety analyses. This study is registered with ClinicalTrials.gov, number NCT00553410, and EudraCT, number 2007-001370-88; and long-term follow-up of patients is ongoing.
FINDINGS
Between Dec 5, 2007, and Oct 8, 2012, 4884 women were enrolled and randomised after exclusion of patients at a non-adherent centre, found to have inadequate documentation of informed consent, immediately withdrew consent, or randomly assigned to intervention groups in error. 4851 women comprised the intention-to-treat population that compared extended intermittent letrozole use (n=2425) with continuous letrozole use (n=2426). After a median follow-up of 60 months (IQR 53-72), disease-free survival was 85·8% (95% CI 84·2-87·2) in the intermittent letrozole group compared with 87·5% (86·0-88·8) in the continuous letrozole group (hazard ratio 1·08, 95% CI 0·93-1·26; p=0·31). Adverse events were reported as expected and were similar between the two groups. The most common grade 3-5 adverse events were hypertension (584 [24%] of 2417 in the intermittent letrozole group vs 517 [21%] of 2411 in the continuous letrozole group) and arthralgia (136 [6%] vs 151 [6%]). 54 patients (24 [1%] in the intermittent letrozole group and 30 [1%] in the continuous letrozole group) had grade 3-5 CNS cerebrovascular ischaemia, 16 (nine [<1%] vs seven [<1%]) had grade 3-5 CNS haemorrhage, and 40 (19 [1%] vs 21 [1%]) had grade 3-5 cardiac ischaemia. In total, 23 (<1%) of 4851 patients died while on trial treatment (13 [<1%] of 2417 patients in the intermittent letrozole group vs ten [<1%] of 2411 in the continuous letrozole group).
INTERPRETATION
In postmenopausal women with hormone receptor-positive breast cancer, extended use of intermittent letrozole did not improve disease-free survival compared with continuous use of letrozole. An alternative schedule of extended adjuvant endocrine therapy with letrozole, including intermittent administration, might be feasible and the results of the SOLE trial support the safety of temporary treatment breaks in selected patients who might require them.
FUNDING
Novartis and the International Breast Cancer Study Group.
中文翻译:

绝经后乳腺癌(SOLE)的辅助辅助间歇性来曲唑与连续来曲唑的比较:一项多中心,开放标签,随机,3期试验。
背景技术在乳腺癌的动物模型中,可以通过停药和重新引入来曲唑来逆转对来曲唑的连续使用的耐药性。因此,我们假设与绝经后妇女连续使用来曲唑相比,长期间歇性使用来曲唑将改善乳腺癌的预后。方法我们在22个国家/地区的240个中心(学术,初级,二级和三级护理中心)进行了多中心,开放标签,随机,平行,3期SOLE试验。我们招募了任何年龄,患有激素受体阳性,淋巴结阳性和可手术的乳腺癌的绝经后妇女,她们接受了局部治疗(有或没有放射疗法的手术),并已完成4-6年的辅助内分泌治疗。在入组时,他们必须在临床上没有乳腺癌,并且在随机分配之前的任何时间都没有复发疾病的迹象。我们将妇女(1:1)随机分配至连续使用来曲唑(2·5 mg /天,口服5年)或间歇使用来曲唑(2·5 mg /天,口服9个月,然后连续3个月)的治疗组。 1-4年的月度休息时间,然后在5年的所有12个月中每天2·5毫克/天。随机化由主要研究者或指定人员通过国际乳腺癌研究组基于互联网的系统在各个中心进行,并根据既往内分泌治疗的类型进行分层(仅芳香化酶抑制剂对选择性雌激素受体调节剂对两种疗法),并使用排列的块大小为4,并实现机构平衡。没有人被掩盖在治疗任务上。主要终点为无病生存期,采用分层对数秩检验通过意向治疗原则进行分析。在安全性分析中纳入了在意向治疗人群中在试验参与期间开始进行方案治疗的所有患者。该研究已在ClinicalTrials.gov注册,编号为NCT00553410,在EudraCT注册为2007-001370-88。并且对患者进行长期随访。结果在2007年12月5日至2012年10月8日期间,有4884名妇女参加了研究,并在非依从性中心排除患者,发现知情同意书证据不足,立即撤回同意或随机分配至干预组后随机分组。错误。有意向治疗的人群共有4851名妇女,他们将长期间歇性来曲唑使用(n = 2425)与连续来曲唑使用(n = 2426)进行了比较。经过60个月的中位随访(IQR 53-72),间歇性来曲唑组的无病生存率为85·8%(95%CI 84·2-87·2),相比之下,无病生存率为87·5%(86)连续来曲唑组中(·0-88·8)(危险比1·08,95%CI 0·93-1·26; p = 0·31)。据报道,不良事件在两组之间相似。最常见的3-5级不良事件是高血压(间歇性来曲唑组为2417的584 [24%],而连续来曲唑组为2411的517 [21%])和关节痛(136 [6%]对151 [6] %])。54例(间歇性来曲唑组为24 [1%],连续来曲唑组为30 [1%])患有3-5级CNS脑血管缺血,16例(9 [< 3-5级中枢神经系统出血有1%]比7 [<1%]),而3-5级心脏缺血有40(19 [1%]比21 [1%])。总共有4851例患者中有23例(<1%)在接受试验治疗时死亡(间歇性来曲唑组2417例患者中有13例[<1%],而连续的来曲唑组2411例中有10例[<1%])。解释在绝经后患有激素受体阳性乳腺癌的妇女中,与持续使用来曲唑相比,长期使用间歇性来曲唑不能改善无病生存期。使用来曲唑扩大辅助内分泌治疗的替代方案,包括间歇给药,可能是可行的,SOLE试验的结果支持了可能需要这些治疗的部分患者暂时停药的安全性。资助诺华和国际乳腺癌研究小组。
更新日期:2017-12-31
中文翻译:

绝经后乳腺癌(SOLE)的辅助辅助间歇性来曲唑与连续来曲唑的比较:一项多中心,开放标签,随机,3期试验。
背景技术在乳腺癌的动物模型中,可以通过停药和重新引入来曲唑来逆转对来曲唑的连续使用的耐药性。因此,我们假设与绝经后妇女连续使用来曲唑相比,长期间歇性使用来曲唑将改善乳腺癌的预后。方法我们在22个国家/地区的240个中心(学术,初级,二级和三级护理中心)进行了多中心,开放标签,随机,平行,3期SOLE试验。我们招募了任何年龄,患有激素受体阳性,淋巴结阳性和可手术的乳腺癌的绝经后妇女,她们接受了局部治疗(有或没有放射疗法的手术),并已完成4-6年的辅助内分泌治疗。在入组时,他们必须在临床上没有乳腺癌,并且在随机分配之前的任何时间都没有复发疾病的迹象。我们将妇女(1:1)随机分配至连续使用来曲唑(2·5 mg /天,口服5年)或间歇使用来曲唑(2·5 mg /天,口服9个月,然后连续3个月)的治疗组。 1-4年的月度休息时间,然后在5年的所有12个月中每天2·5毫克/天。随机化由主要研究者或指定人员通过国际乳腺癌研究组基于互联网的系统在各个中心进行,并根据既往内分泌治疗的类型进行分层(仅芳香化酶抑制剂对选择性雌激素受体调节剂对两种疗法),并使用排列的块大小为4,并实现机构平衡。没有人被掩盖在治疗任务上。主要终点为无病生存期,采用分层对数秩检验通过意向治疗原则进行分析。在安全性分析中纳入了在意向治疗人群中在试验参与期间开始进行方案治疗的所有患者。该研究已在ClinicalTrials.gov注册,编号为NCT00553410,在EudraCT注册为2007-001370-88。并且对患者进行长期随访。结果在2007年12月5日至2012年10月8日期间,有4884名妇女参加了研究,并在非依从性中心排除患者,发现知情同意书证据不足,立即撤回同意或随机分配至干预组后随机分组。错误。有意向治疗的人群共有4851名妇女,他们将长期间歇性来曲唑使用(n = 2425)与连续来曲唑使用(n = 2426)进行了比较。经过60个月的中位随访(IQR 53-72),间歇性来曲唑组的无病生存率为85·8%(95%CI 84·2-87·2),相比之下,无病生存率为87·5%(86)连续来曲唑组中(·0-88·8)(危险比1·08,95%CI 0·93-1·26; p = 0·31)。据报道,不良事件在两组之间相似。最常见的3-5级不良事件是高血压(间歇性来曲唑组为2417的584 [24%],而连续来曲唑组为2411的517 [21%])和关节痛(136 [6%]对151 [6] %])。54例(间歇性来曲唑组为24 [1%],连续来曲唑组为30 [1%])患有3-5级CNS脑血管缺血,16例(9 [< 3-5级中枢神经系统出血有1%]比7 [<1%]),而3-5级心脏缺血有40(19 [1%]比21 [1%])。总共有4851例患者中有23例(<1%)在接受试验治疗时死亡(间歇性来曲唑组2417例患者中有13例[<1%],而连续的来曲唑组2411例中有10例[<1%])。解释在绝经后患有激素受体阳性乳腺癌的妇女中,与持续使用来曲唑相比,长期使用间歇性来曲唑不能改善无病生存期。使用来曲唑扩大辅助内分泌治疗的替代方案,包括间歇给药,可能是可行的,SOLE试验的结果支持了可能需要这些治疗的部分患者暂时停药的安全性。资助诺华和国际乳腺癌研究小组。











































 京公网安备 11010802027423号
京公网安备 11010802027423号