当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phosphorylation versus O-GlcNAcylation: Computational Insights into the Differential Influences of the Two Competitive Post-Translational Modifications
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.jpcb.7b08790 Lata Rani 1 , Sairam S. Mallajosyula 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.jpcb.7b08790 Lata Rani 1 , Sairam S. Mallajosyula 1
Affiliation
|
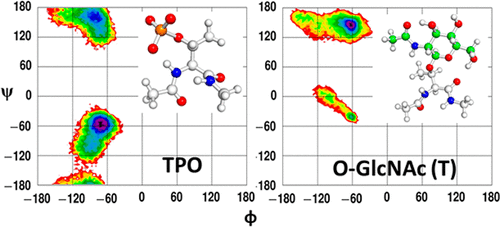
Phosphorylation and O-GlcNAcylation are rapidly cycling intracellular protein post-translational modifications (PTMs) that can compete for the same serine (S) and threonine (T) sites. Limited crystal structure information is available on the direct influence of these PTMs on the underlying protein structure, especially for O-GlcNAcylation. NMR and CD studies show that these competitive-PTMs can have the same or differential influence on the overall secondary structure. In Tau derived peptide fragments, it was found that phosphorylation stabilized PPII conformations while O-GlcNAcylation destabilized the same. In the absence of substantial structural information, we have performed a systematic computational study utilizing PDB analysis, QM calculations, and MD simulations to identify key structural trends upon PTM. Our analysis of the limited PDB data set revealed conformational shifts from PPII to α-helical geometry upon serine phosphorylation and in the opposite direction, from α-helical to PPII geometry upon threonine phosphorylation. Gas phase QM calculations covering the complete Ramachandran ϕ/ψ space using model native, phosphorylated, and O-GlcNAcylated dipeptide systems revealed preferences toward α-helical conformations. However, the major structural transitions were observed in the MD simulations upon the inclusion of solvation. The model dipeptide simulations revealed a preference for PPII and α-helical conformations for phosphorylated serine and threonine, while O-GlcNAcylated dipeptides exhibited a complete shift toward extended conformations, β-sheet and PPII, disfavoring the α-helical conformation. For the Baldwin α-helix simulations, it was found that both phosphorylation and O-GlcNAcylation destabilized the helix; however, the destabilization was governed by H-bonding and electrostatic interactions in the former, while the latter was controlled by hydrophobic collapse and steric interactions. The presence of lysine in close proximity of phosphate leads to potentially stable salt bridge interactions, which can influence the structure on the basis of the relative placement of the lysine with respect to the PTM site. Similar strong lysine–phosphate contacts were observed in the model Tau peptides, which steers the conformations toward PPII geometries, highlighting the direct influence of the PTM on function.
中文翻译:

磷酸化与O-GlcNAcylation:两个竞争性翻译后修饰的差异影响的计算见解。
磷酸化和O-GlcNAcylation是快速循环的细胞内蛋白翻译后修饰(PTM),可以竞争相同的丝氨酸(S)和苏氨酸(T)位点。有关这些PTM对基础蛋白质结构的直接影响(尤其是对于O-GlcNAcylation)的直接影响,有限的晶体结构信息是可用的。NMR和CD研究表明,这些竞争性PTM对总体二级结构可能具有相同或不同的影响。在Tau衍生的肽片段中,发现磷酸化可以稳定PPII构象,而O-GlcNAcylation可以使PPII构象不稳定。在缺乏实质性结构信息的情况下,我们已经进行了系统的计算研究,利用PDB分析,QM计算和MD模拟来确定PTM上的关键结构趋势。我们对有限的PDB数据集的分析显示,在丝氨酸磷酸化后,从PPII构象转变为α-螺旋几何构型,而在苏氨酸磷酸化时,从α-螺旋构象构象转变为PPII几何构型。气相QM计算覆盖了整个Ramachandran ϕ /ψ空间,使用模型天然,磷酸化和O-GlcNAcy酰化二肽系统进行了建模,揭示了人们对α-螺旋构象的偏爱。然而,在包含溶剂化的MD模拟中观察到主要的结构转变。模型二肽模拟显示,磷酸化丝氨酸和苏氨酸偏爱PPII和α-螺旋构象,而O-GlcNA酰化二肽显示出向扩展构象,β-折叠和PPII的完全转变,不利于α-螺旋构象。对于Baldwinα-螺旋模拟,发现磷酸化和O-GlcNAcylation都使螺旋不稳定。然而,在前者中,不稳定是由氢键和静电相互作用控制的,而后者则由疏水性塌陷和空间相互作用控制。赖氨酸在磷酸盐附近的存在会导致潜在的稳定的盐桥相互作用,这可能会基于赖氨酸相对于PTM位点的相对位置影响结构。在Tau肽模型中观察到了类似的赖氨酸-磷酸强烈接触,这使构象趋向于PPII几何结构,从而突出了PTM对功能的直接影响。前者的失稳主要由氢键和静电相互作用控制,而后者则由疏水性塌陷和空间相互作用控制。赖氨酸在磷酸盐附近的存在会导致潜在的稳定的盐桥相互作用,这可能会基于赖氨酸相对于PTM位点的相对位置影响结构。在Tau肽模型中观察到了类似的赖氨酸-磷酸强烈接触,这使构象趋向于PPII几何结构,突出了PTM对功能的直接影响。前者的失稳主要由氢键和静电相互作用控制,而后者则由疏水性塌陷和空间相互作用控制。赖氨酸在磷酸盐附近的存在会导致潜在的稳定的盐桥相互作用,这可能会基于赖氨酸相对于PTM位点的相对位置影响结构。在Tau肽模型中观察到了类似的赖氨酸-磷酸强烈接触,这使构象趋向于PPII几何结构,突出了PTM对功能的直接影响。它可以基于赖氨酸相对于PTM位置的相对位置影响结构。在Tau肽模型中观察到了类似的赖氨酸-磷酸强烈接触,这使构象趋向于PPII几何结构,突出了PTM对功能的直接影响。它可以基于赖氨酸相对于PTM位置的相对位置影响结构。在Tau肽模型中观察到了类似的赖氨酸-磷酸强烈接触,这使构象趋向于PPII几何结构,突出了PTM对功能的直接影响。
更新日期:2017-11-19
中文翻译:

磷酸化与O-GlcNAcylation:两个竞争性翻译后修饰的差异影响的计算见解。
磷酸化和O-GlcNAcylation是快速循环的细胞内蛋白翻译后修饰(PTM),可以竞争相同的丝氨酸(S)和苏氨酸(T)位点。有关这些PTM对基础蛋白质结构的直接影响(尤其是对于O-GlcNAcylation)的直接影响,有限的晶体结构信息是可用的。NMR和CD研究表明,这些竞争性PTM对总体二级结构可能具有相同或不同的影响。在Tau衍生的肽片段中,发现磷酸化可以稳定PPII构象,而O-GlcNAcylation可以使PPII构象不稳定。在缺乏实质性结构信息的情况下,我们已经进行了系统的计算研究,利用PDB分析,QM计算和MD模拟来确定PTM上的关键结构趋势。我们对有限的PDB数据集的分析显示,在丝氨酸磷酸化后,从PPII构象转变为α-螺旋几何构型,而在苏氨酸磷酸化时,从α-螺旋构象构象转变为PPII几何构型。气相QM计算覆盖了整个Ramachandran ϕ /ψ空间,使用模型天然,磷酸化和O-GlcNAcy酰化二肽系统进行了建模,揭示了人们对α-螺旋构象的偏爱。然而,在包含溶剂化的MD模拟中观察到主要的结构转变。模型二肽模拟显示,磷酸化丝氨酸和苏氨酸偏爱PPII和α-螺旋构象,而O-GlcNA酰化二肽显示出向扩展构象,β-折叠和PPII的完全转变,不利于α-螺旋构象。对于Baldwinα-螺旋模拟,发现磷酸化和O-GlcNAcylation都使螺旋不稳定。然而,在前者中,不稳定是由氢键和静电相互作用控制的,而后者则由疏水性塌陷和空间相互作用控制。赖氨酸在磷酸盐附近的存在会导致潜在的稳定的盐桥相互作用,这可能会基于赖氨酸相对于PTM位点的相对位置影响结构。在Tau肽模型中观察到了类似的赖氨酸-磷酸强烈接触,这使构象趋向于PPII几何结构,从而突出了PTM对功能的直接影响。前者的失稳主要由氢键和静电相互作用控制,而后者则由疏水性塌陷和空间相互作用控制。赖氨酸在磷酸盐附近的存在会导致潜在的稳定的盐桥相互作用,这可能会基于赖氨酸相对于PTM位点的相对位置影响结构。在Tau肽模型中观察到了类似的赖氨酸-磷酸强烈接触,这使构象趋向于PPII几何结构,突出了PTM对功能的直接影响。前者的失稳主要由氢键和静电相互作用控制,而后者则由疏水性塌陷和空间相互作用控制。赖氨酸在磷酸盐附近的存在会导致潜在的稳定的盐桥相互作用,这可能会基于赖氨酸相对于PTM位点的相对位置影响结构。在Tau肽模型中观察到了类似的赖氨酸-磷酸强烈接触,这使构象趋向于PPII几何结构,突出了PTM对功能的直接影响。它可以基于赖氨酸相对于PTM位置的相对位置影响结构。在Tau肽模型中观察到了类似的赖氨酸-磷酸强烈接触,这使构象趋向于PPII几何结构,突出了PTM对功能的直接影响。它可以基于赖氨酸相对于PTM位置的相对位置影响结构。在Tau肽模型中观察到了类似的赖氨酸-磷酸强烈接触,这使构象趋向于PPII几何结构,突出了PTM对功能的直接影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号