当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Heptavalent Actinide Tetroxides NpO4– and PuO4–: Oxidation of Pu(V) to Pu(VII) by Adding an Electron to PuO4
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.jpca.7b09721 John K. Gibson 1 , Wibe A. de Jong 1 , Phuong D. Dau 1 , Yu Gong 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.jpca.7b09721 John K. Gibson 1 , Wibe A. de Jong 1 , Phuong D. Dau 1 , Yu Gong 1
Affiliation
|
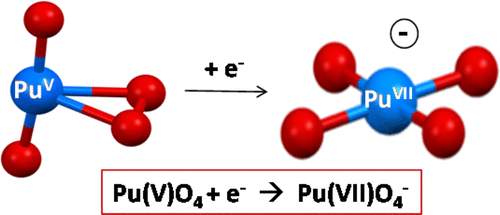
The highest known actinide oxidation states are Np(VII) and Pu(VII), both of which have been identified in solution and solid compounds. Recently a molecular Np(VII) complex, NpO3(NO3)2–, was prepared and characterized in the gas phase. In accord with the lower stability of heptavalent Pu, no Pu(VII) molecular species has been identified. Reported here are the gas-phase syntheses and characterizations of NpO4– and PuO4–. Reactivity studies and density functional theory computations indicate the heptavalent metal oxidation state in both. This is the first instance of Pu(VII) in the absence of stabilizing effects due to condensed phase solvation or crystal fields. The results indicate that addition of an electron to neutral PuO4, which has a computed electron affinity of 2.56 eV, counterintuitively results in oxidation of Pu(V) to Pu(VII), concomitant with superoxide reduction.
中文翻译:

七价锕Tetroxides NPO 4 -和二氧化钚4 - :通过加入电子至的PuO浦(V)到浦(VII)氧化4
已知最高的act系元素氧化态是Np(VII)和Pu(VII),这两种化合物都已在溶液和固体化合物中鉴定出来。最近的分子的NP(VII)络合物,NPO 3(NO 3)2 - ,制备和表征在气相中。与七价Pu的较低稳定性相一致,尚未鉴定出Pu(VII)分子种类。这里报道的是NpO 4 –和PuO 4 –的气相合成和表征。。反应性研究和密度泛函理论计算表明两者中的七价金属氧化态。这是Pu(VII)的第一个实例,由于凝聚相溶剂化或晶体场而没有稳定作用。结果表明,将电子添加到中性PuO 4中(计算得出的电子亲和力为2.56 eV)反直觉地导致Pu(V)氧化为Pu(VII),并伴随超氧化物还原。
更新日期:2017-11-19
中文翻译:

七价锕Tetroxides NPO 4 -和二氧化钚4 - :通过加入电子至的PuO浦(V)到浦(VII)氧化4
已知最高的act系元素氧化态是Np(VII)和Pu(VII),这两种化合物都已在溶液和固体化合物中鉴定出来。最近的分子的NP(VII)络合物,NPO 3(NO 3)2 - ,制备和表征在气相中。与七价Pu的较低稳定性相一致,尚未鉴定出Pu(VII)分子种类。这里报道的是NpO 4 –和PuO 4 –的气相合成和表征。。反应性研究和密度泛函理论计算表明两者中的七价金属氧化态。这是Pu(VII)的第一个实例,由于凝聚相溶剂化或晶体场而没有稳定作用。结果表明,将电子添加到中性PuO 4中(计算得出的电子亲和力为2.56 eV)反直觉地导致Pu(V)氧化为Pu(VII),并伴随超氧化物还原。











































 京公网安备 11010802027423号
京公网安备 11010802027423号