当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nanotopographic Regulation of Human Mesenchymal Stem Cell Osteogenesis
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acsami.7b16314 Weiyi Qian 1 , Lanqi Gong 1 , Xin Cui 1 , Zijing Zhang 1 , Apratim Bajpai 1 , Chao Liu 1, 2 , Alesha B. Castillo 1, 2 , Jeremy C. M. Teo 3 , Weiqiang Chen 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acsami.7b16314 Weiyi Qian 1 , Lanqi Gong 1 , Xin Cui 1 , Zijing Zhang 1 , Apratim Bajpai 1 , Chao Liu 1, 2 , Alesha B. Castillo 1, 2 , Jeremy C. M. Teo 3 , Weiqiang Chen 1
Affiliation
|
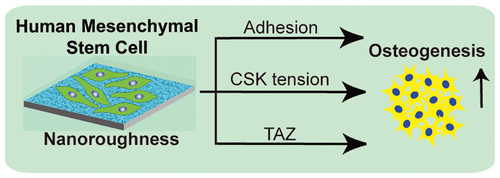
Mesenchymal stem cell (MSC) differentiation can be manipulated by nanotopographic interface providing a unique strategy to engineering stem cell therapy and circumventing complex cellular reprogramming. However, our understanding of the nanotopographic–mechanosensitive properties of MSCs and the underlying biophysical linkage of the nanotopography-engineered stem cell to directed commitment remains elusive. Here, we show that osteogenic differentiation of human MSCs (hMSCs) can be largely promoted using our nanoengineered topographic glass substrates in the absence of dexamethasone, a key exogenous factor for osteogenesis induction. We demonstrate that hMSCs sense and respond to surface nanotopography, through modulation of adhesion, cytoskeleton tension, and nuclear activation of TAZ (transcriptional coactivator with PDZ-binding motif), a transcriptional modulator of hMSCs. Our findings demonstrate the potential of nanotopographic surfaces as noninvasive tools to advance cell-based therapies for bone engineering and highlight the origin of biophysical response of hMSC to nanotopography.
中文翻译:

人间充质干细胞成骨的纳米形貌调控。
间充质干细胞(MSC)的分化可以通过纳米形貌界面来控制,从而为工程化干细胞治疗和规避复杂的细胞重编程提供了独特的策略。但是,我们对MSCs的纳米形貌力学敏感特性以及纳米形貌工程化干细胞与定向承诺之间潜在的生物物理联系的理解仍然难以捉摸。在这里,我们表明,在不存在地塞米松(诱导成骨的关键外源因子)的情况下,使用我们的纳米工程地形玻璃基板可以大大促进人类MSC(hMSC)的成骨分化。我们证明hMSCs通过调节粘附力,细胞骨架张力和TAZ的核激活(具有PDZ结合基序的转录共激活剂)来感知并响应表面纳米形貌,hMSC的转录调节剂。我们的发现证明了纳米形貌表面作为非侵入性工具的潜力,可以促进基于细胞的骨工程疗法的发展,并突出了hMSC对纳米形貌的生物物理反应的起源。
更新日期:2017-11-19
中文翻译:

人间充质干细胞成骨的纳米形貌调控。
间充质干细胞(MSC)的分化可以通过纳米形貌界面来控制,从而为工程化干细胞治疗和规避复杂的细胞重编程提供了独特的策略。但是,我们对MSCs的纳米形貌力学敏感特性以及纳米形貌工程化干细胞与定向承诺之间潜在的生物物理联系的理解仍然难以捉摸。在这里,我们表明,在不存在地塞米松(诱导成骨的关键外源因子)的情况下,使用我们的纳米工程地形玻璃基板可以大大促进人类MSC(hMSC)的成骨分化。我们证明hMSCs通过调节粘附力,细胞骨架张力和TAZ的核激活(具有PDZ结合基序的转录共激活剂)来感知并响应表面纳米形貌,hMSC的转录调节剂。我们的发现证明了纳米形貌表面作为非侵入性工具的潜力,可以促进基于细胞的骨工程疗法的发展,并突出了hMSC对纳米形貌的生物物理反应的起源。











































 京公网安备 11010802027423号
京公网安备 11010802027423号