Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On-Surface Annulation Reaction Cascade for the Selective Synthesis of Diindenopyrene
ACS Nano ( IF 15.8 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acsnano.7b06459 Frank Eisenhut 1 , Thomas Lehmann 1 , Andreas Viertel 2 , Dmitry Skidin 1 , Justus Krüger 1 , Seddigheh Nikipar 1 , Dmitry A. Ryndyk 1, 3 , Christian Joachim 4 , Stefan Hecht 2 , Francesca Moresco 1 , Gianaurelio Cuniberti 1, 5
ACS Nano ( IF 15.8 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acsnano.7b06459 Frank Eisenhut 1 , Thomas Lehmann 1 , Andreas Viertel 2 , Dmitry Skidin 1 , Justus Krüger 1 , Seddigheh Nikipar 1 , Dmitry A. Ryndyk 1, 3 , Christian Joachim 4 , Stefan Hecht 2 , Francesca Moresco 1 , Gianaurelio Cuniberti 1, 5
Affiliation
|
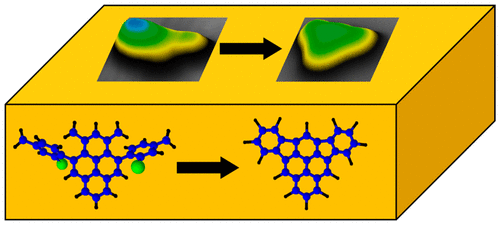
We investigated the thermally induced on-surface cyclization of 4,10-bis(2′-bromo-4′-methylphenyl)-1,3-dimethylpyrene to form the previously unknown, nonalternant polyaromatic hydrocarbon diindeno[1,2,3-cd:1′,2′,3′-mn]pyrene on Au(111) using scanning tunneling microscopy and spectroscopy. The observed unimolecular reaction involves thermally induced debromination followed by selective ring closure to fuse the neighboring benzene moieties via a five-membered ring. The structure of the product has been verified experimentally as well as theoretically. Our results demonstrate that on-surface reactions give rise to unusual chemical reactivities and selectivities and provide access to nonalternant polyaromatic molecules.
中文翻译:

选择性合成二茚并py的表面环空反应级联
我们研究了4,10-双(2'-溴-4'-甲基苯基)-1,3-二甲基py的热诱导表面环化反应,以形成先前未知的非替代性聚芳烃二茚并[1,2,3-cd使用扫描隧道显微镜和光谱法在Au(111)上合成:1',2',3'-mn] py。观察到的单分子反应涉及热诱导的脱溴作用,然后进行选择性的闭环,以通过五元环融合相邻的苯部分。产品的结构已通过实验和理论验证。我们的结果表明,表面反应会引起异常的化学反应性和选择性,并提供了非交替的多芳族分子的通道。
更新日期:2017-11-19
中文翻译:

选择性合成二茚并py的表面环空反应级联
我们研究了4,10-双(2'-溴-4'-甲基苯基)-1,3-二甲基py的热诱导表面环化反应,以形成先前未知的非替代性聚芳烃二茚并[1,2,3-cd使用扫描隧道显微镜和光谱法在Au(111)上合成:1',2',3'-mn] py。观察到的单分子反应涉及热诱导的脱溴作用,然后进行选择性的闭环,以通过五元环融合相邻的苯部分。产品的结构已通过实验和理论验证。我们的结果表明,表面反应会引起异常的化学反应性和选择性,并提供了非交替的多芳族分子的通道。











































 京公网安备 11010802027423号
京公网安备 11010802027423号