当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploiting Alkylquinone Tautomerization for the Total Synthesis of Calothrixin A and B
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.joc.7b02101 Luis M. Mori-Quiroz 1 , Madeline M. Dekarske 1 , Austin B. Prinkki 1 , Michael D. Clift 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.joc.7b02101 Luis M. Mori-Quiroz 1 , Madeline M. Dekarske 1 , Austin B. Prinkki 1 , Michael D. Clift 1
Affiliation
|
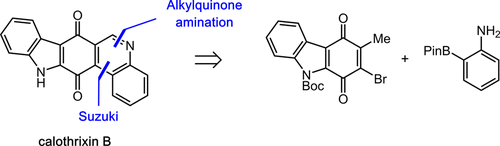
The pentacyclic alkaloid calothrixin B (1) has been synthesized in 5 steps from murrayaquinone A (9). The key step involved the union of boryl aniline 31 with brominated murrayaquinone A (26). In this transformation, alkylquinone 26 undergoes tautomerization to a quinone methide, which is intercepted by boryl aniline 31 to forge a new C–N bond. An intramolecular Suzuki coupling, followed by dehydrogenative aromatization, completed the synthesis of calothrixin B. Subsequent N-oxidation of calothrixin B delivered calothrixin A. The successful synthesis of these alkaloids and the challenges that led to the development of the final synthesis plan are reported herein.
中文翻译:

利用烷基醌互变异构法合成Calothrixin A和B
五环生物碱calothrixin B(1)已从Murrayaquinone A(9)分5个步骤合成。关键步骤涉及将硼基苯胺31与溴化的murrayaquinone A(26)结合。在这种转化过程中,烷基醌26进行互变异构化为甲基化醌,然后被硼基苯胺31截获,从而形成一个新的C–N键。分子内的Suzuki偶联,然后进行脱氢芳构化,完成了calothrixin B的合成。随后,calothrixin B的N-氧化作用递送了calothrixinA。本文报道了这些生物碱的成功合成及其带来的挑战,这些挑战导致了最终合成计划的制定。
更新日期:2017-11-19
中文翻译:

利用烷基醌互变异构法合成Calothrixin A和B
五环生物碱calothrixin B(1)已从Murrayaquinone A(9)分5个步骤合成。关键步骤涉及将硼基苯胺31与溴化的murrayaquinone A(26)结合。在这种转化过程中,烷基醌26进行互变异构化为甲基化醌,然后被硼基苯胺31截获,从而形成一个新的C–N键。分子内的Suzuki偶联,然后进行脱氢芳构化,完成了calothrixin B的合成。随后,calothrixin B的N-氧化作用递送了calothrixinA。本文报道了这些生物碱的成功合成及其带来的挑战,这些挑战导致了最终合成计划的制定。











































 京公网安备 11010802027423号
京公网安备 11010802027423号