当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
One-Pot Synthesis of α-Carbonyl Bicyclic Furans via a Sequential Diels–Alder/5-Exo-Dig Cyclization/Oxidation Reaction
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.joc.7b02479 Khagendra B. Hamal 1 , Wesley A. Chalifoux 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.joc.7b02479 Khagendra B. Hamal 1 , Wesley A. Chalifoux 1
Affiliation
|
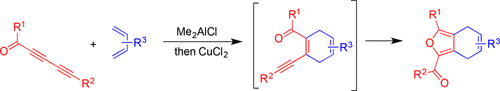
A new one-pot approach to construct α-carbonyl bicyclic furans from easily accessible diynones is described. This reaction sequence proceeds via a Diels–Alder cycloaddition reaction catalyzed by dimethylaluminum chloride followed by a 5-exo-dig cyclization/oxidation reaction catalyzed by copper(II) chloride. This methodology generates α-carbonyl-functionalized dihydroisobenzofuran derivatives in good to excellent yields.
中文翻译:

通过顺序Diels–Alder / 5-Exo-Dig环化/氧化反应一锅法合成α-羰基双环呋喃
描述了一种新的一锅法,从容易获得的二炔酮构造α-羰基双环呋喃。该反应顺序是通过氯化二甲基铝催化的狄尔斯-阿尔德环加成反应进行的,然后是氯化铜(II)催化的5-exo-dig环化/氧化反应。该方法以良好至优异的产率产生α-羰基官能化的二氢异苯并呋喃衍生物。
更新日期:2017-11-19
中文翻译:

通过顺序Diels–Alder / 5-Exo-Dig环化/氧化反应一锅法合成α-羰基双环呋喃
描述了一种新的一锅法,从容易获得的二炔酮构造α-羰基双环呋喃。该反应顺序是通过氯化二甲基铝催化的狄尔斯-阿尔德环加成反应进行的,然后是氯化铜(II)催化的5-exo-dig环化/氧化反应。该方法以良好至优异的产率产生α-羰基官能化的二氢异苯并呋喃衍生物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号