当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cysteine Linkages Accelerate Electron Flow through Tetra-Heme Protein STC
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/jacs.7b08831 Xiuyun Jiang 1 , Zdenek Futera 1 , Md. Ehesan Ali 1 , Fruzsina Gajdos 1 , Guido F. von Rudorff 1 , Antoine Carof 1 , Marian Breuer 1 , Jochen Blumberger 1, 2
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/jacs.7b08831 Xiuyun Jiang 1 , Zdenek Futera 1 , Md. Ehesan Ali 1 , Fruzsina Gajdos 1 , Guido F. von Rudorff 1 , Antoine Carof 1 , Marian Breuer 1 , Jochen Blumberger 1, 2
Affiliation
|
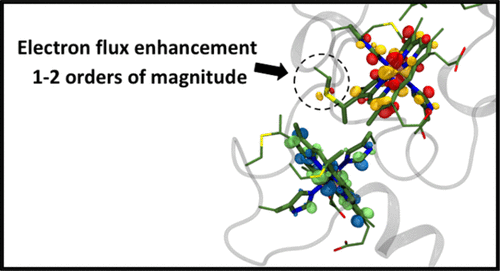
Multi-heme proteins have attracted much attention recently due to their prominent role in mediating extracellular electron transport (ET), but one of their key fundamental properties, the rate constants for ET between the constituent heme groups, have so far evaded experimental determination. Here we report the set of heme–heme theoretical ET rate constants that define electron flow in the tetra-heme protein STC by combining a novel projector-operator diabatization approach for electronic coupling calculation with molecular dynamics simulation of ET free energies. On the basis of our calculations, we find that the protein limited electron flux through STC in the thermodynamic downhill direction (heme 1→4) is ∼3 × 106 s–1. We find that cysteine linkages inserting in the space between the two terminal heme pairs 1-2 and 3-4 significantly enhance the overall electron flow, by a factor of about 37, due to weak mixing of the sulfur 3p orbital with the Fe-heme d orbitals. While the packing density model, and to a higher degree, the pathway model of biological ET partly capture the predicted rate enhancements, our study highlights the importance of the atomistic and chemical nature of the tunneling medium at short biological tunneling distances. Cysteine linkages are likely to enhance electron flow also in the larger deca-heme proteins MtrC and MtrF, where heme–heme motifs with sub-optimal edge-to-edge distances are used to shuttle electrons in multiple directions.
中文翻译:

半胱氨酸键加速通过四血红素蛋白STC的电子流动
多血红素蛋白由于其在介导细胞外电子转运(ET)中的突出作用,近来备受关注,但是到目前为止,它们的关键基本特性之一,即组成血红素基团之间的ET速率常数,一直未能通过实验确定。在这里,我们通过结合新颖的投影仪-算子绝热方法进行电子耦合计算与ET自由能的分子动力学模拟,报告了定义四血红素蛋白STC中电子流的血红素-血红素理论ET速率常数。根据我们的计算,我们发现在热力学下坡方向(血红素1→4)通过STC的蛋白质限制电子通量约为3×10 6 s –1。我们发现,由于硫3p轨道与铁血红素的弱混合,插入两个末端血红素对1-2和3-4之间的空间中的半胱氨酸键显着提高了总电子流,约为37倍。 d轨道。尽管堆积密度模型以及更高程度上的生物ET路径模型部分捕获了预测的速率提高,但我们的研究强调了在短的生物隧穿距离下隧穿介质的原子性和化学性质的重要性。半胱氨酸键也可能会在较大的十血红素蛋白MtrC和MtrF中增强电子流动,其中具有次优边缘到边缘距离的血红素-血红素基序被用来在多个方向上穿梭电子。
更新日期:2017-11-19
中文翻译:

半胱氨酸键加速通过四血红素蛋白STC的电子流动
多血红素蛋白由于其在介导细胞外电子转运(ET)中的突出作用,近来备受关注,但是到目前为止,它们的关键基本特性之一,即组成血红素基团之间的ET速率常数,一直未能通过实验确定。在这里,我们通过结合新颖的投影仪-算子绝热方法进行电子耦合计算与ET自由能的分子动力学模拟,报告了定义四血红素蛋白STC中电子流的血红素-血红素理论ET速率常数。根据我们的计算,我们发现在热力学下坡方向(血红素1→4)通过STC的蛋白质限制电子通量约为3×10 6 s –1。我们发现,由于硫3p轨道与铁血红素的弱混合,插入两个末端血红素对1-2和3-4之间的空间中的半胱氨酸键显着提高了总电子流,约为37倍。 d轨道。尽管堆积密度模型以及更高程度上的生物ET路径模型部分捕获了预测的速率提高,但我们的研究强调了在短的生物隧穿距离下隧穿介质的原子性和化学性质的重要性。半胱氨酸键也可能会在较大的十血红素蛋白MtrC和MtrF中增强电子流动,其中具有次优边缘到边缘距离的血红素-血红素基序被用来在多个方向上穿梭电子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号