Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation and Capacity-Fading Investigation of Polymer-Derived Silicon Carbonitride Anode for Lithium-Ion Battery
ACS Omega ( IF 3.7 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acsomega.7b01462 Yan Feng 1 , Shuming Dou , Yuzhen Wei , Yuliang Zhang , Xiangyun Song 1 , Xifei Li 2 , Vincent S. Battaglia 1
ACS Omega ( IF 3.7 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acsomega.7b01462 Yan Feng 1 , Shuming Dou , Yuzhen Wei , Yuliang Zhang , Xiangyun Song 1 , Xifei Li 2 , Vincent S. Battaglia 1
Affiliation
|
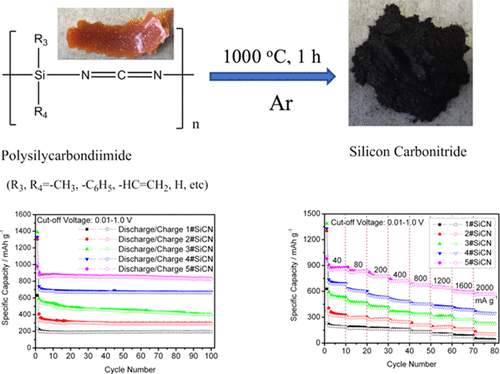
Polymer-derived silicon carbonitride (SiCN) materials have been synthesized via pyrolyzing from five poly(silylcarbondiimide)s with different contents of carbon (labeled as 1–5#). The morphological and structural measurements show that the SiCN materials are mixtures of nanocrystals of SiC, Si3N4, and graphite. The SiCN materials have been used as anodes for lithium-ion batteries. Among the five polymer-derived SiCN materials, 5#SiCN, derived from dichloromethylvinylsilane and di-n-octyldichlorosilane, has the best cycle stability and a high-rate performance at the low cutoff voltage of 0.01–1.0 V. In lithium-ion half-cells, the specific delithiation capacity of 5#SiCN anode still remains at 826.7 mA h g–1 after 100 charge/discharge cycles; it can even deliver the capacity above 550 mA h g–1 at high current densities of 1.6 and 2 A g–1. In lithium-ion full cells, 5#SiCN anode works well with LiNi0.6Co0.2Mn0.2O2 commercial cathode. The outstanding electrochemical performance of 5#SiCN anode is attributed to two factors: (1) the formation of a stable and compact solid electrolyte interface layer on the anode surface anode, which protects the electrode from cracking during the charge/discharge cycle; and (2) a large amount of carbon component and the less Si3N4 phase in the 5#SiCN structure, which provides an electrochemical reactive and conductive environment in the SiCN structure, benefit the lithiation/delithiation process. In addition, we explore the reason for the capacity fading of these SiCN anodes.
中文翻译:

锂离子电池聚合物衍生的碳氮化硅阳极的制备及减容研究
聚合物衍生的碳氮化硅(SiCN)材料是通过热解由五个碳含量不同(标记为1-5#)的聚(甲硅烷基碳二酰亚胺)合成的。形态和结构测量表明,SiCN材料是SiC,Si 3 N 4和石墨的纳米晶体的混合物。该SiCN材料已经被用作锂离子电池的阳极。在五种聚合物衍生的SiCN材料中,由二氯甲基乙烯基硅烷和二正辛基二氯硅烷衍生的5#SiCN在0.01–1.0 V的低截止电压下具有最佳的循环稳定性和高倍率性能。电池,5#SiCN阳极的脱锂能力仍保持在826.7 mA hg –1经过100次充电/放电循环后; 它甚至可以在1.6和2 A g –1的高电流密度下提供550 mA hg –1以上的容量。在锂离子满电池中,5#SiCN阳极与LiNi 0.6 Co 0.2 Mn 0.2 O 2商业阴极可以很好地工作。5#SiCN阳极出色的电化学性能归因于两个因素:(1)在阳极表面阳极上形成稳定而致密的固体电解质界面层,可防止电极在充电/放电循环中破裂。(2)大量的碳组分和较少的Si 3 N 4在SiCN结构中提供电化学反应和导电环境的5#SiCN结构中的N-相有利于锂化/脱锂工艺。另外,我们探索了这些SiCN阳极容量下降的原因。
更新日期:2017-11-17
中文翻译:

锂离子电池聚合物衍生的碳氮化硅阳极的制备及减容研究
聚合物衍生的碳氮化硅(SiCN)材料是通过热解由五个碳含量不同(标记为1-5#)的聚(甲硅烷基碳二酰亚胺)合成的。形态和结构测量表明,SiCN材料是SiC,Si 3 N 4和石墨的纳米晶体的混合物。该SiCN材料已经被用作锂离子电池的阳极。在五种聚合物衍生的SiCN材料中,由二氯甲基乙烯基硅烷和二正辛基二氯硅烷衍生的5#SiCN在0.01–1.0 V的低截止电压下具有最佳的循环稳定性和高倍率性能。电池,5#SiCN阳极的脱锂能力仍保持在826.7 mA hg –1经过100次充电/放电循环后; 它甚至可以在1.6和2 A g –1的高电流密度下提供550 mA hg –1以上的容量。在锂离子满电池中,5#SiCN阳极与LiNi 0.6 Co 0.2 Mn 0.2 O 2商业阴极可以很好地工作。5#SiCN阳极出色的电化学性能归因于两个因素:(1)在阳极表面阳极上形成稳定而致密的固体电解质界面层,可防止电极在充电/放电循环中破裂。(2)大量的碳组分和较少的Si 3 N 4在SiCN结构中提供电化学反应和导电环境的5#SiCN结构中的N-相有利于锂化/脱锂工艺。另外,我们探索了这些SiCN阳极容量下降的原因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号