当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
How Does the Proliferating Cell Nuclear Antigen Modulate Binding Specificity to Multiple Partner Proteins?
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.jcim.7b00171 Hubert Li 1 , Manbir Sandhu 1 , Linda H. Malkas 1 , Robert J. Hickey 1 , Nagarajan Vaidehi 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.jcim.7b00171 Hubert Li 1 , Manbir Sandhu 1 , Linda H. Malkas 1 , Robert J. Hickey 1 , Nagarajan Vaidehi 1
Affiliation
|
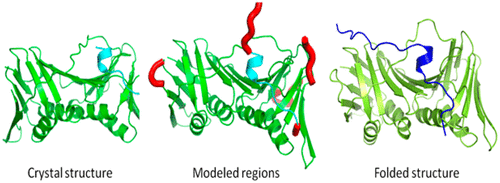
Proliferating cell nuclear antigen (PCNA) is a member of the family of sliding clamp proteins that serves as a clamp during DNA repair, DNA replication, cell cycle control, and multiple forms of chromatin modification. PCNA functions as a homotrimer and complexes with multiple proteins in order to carry out each of these varied functions. PCNA binds to different partner proteins in the same region of its structure, called the “ interdomain connecting loop”, but with different affinities. This interdomain connecting loop is an intrinsically disordered region that takes different conformations when binding to different partner proteins. In this work, we performed all-atom molecular dynamics simulations on PCNA trimer unbound to any partner protein, PCNA bound to peptides from different partner proteins, and PCNA bound to the full Fen 1 protein in two different conformations. Using this massive amount of simulation results, we analyzed whether PCNA in its free trimeric form samples conformations that are similar to those when it is bound to different partner proteins. We observed that PCNA samples many of these peptide-bound conformations even when not bound to the peptides and selects specific conformations when binding to partner proteins. We also identified PCNA–peptide interactions formed in the peptide bound simulation that play a crucial role in complex formation. The calculated binding energies correlate well with the measured binding affinities of various peptides to PCNA. Lastly, we studied the internal dynamics of PCNA and propose a mechanism through which PCNA recruits binding partners. This work highlights the functional role of intrinsically disordered regions in multifunctional proteins such as PCNA.
中文翻译:

增殖细胞核抗原如何调节与多个伴侣蛋白的结合特异性?
增殖细胞核抗原(PCNA)是滑动夹蛋白家族的成员,该蛋白在DNA修复,DNA复制,细胞周期控制和染色质修饰的多种形式中起着钳夹作用。PCNA充当同源三聚体,并与多种蛋白质复合,以执行这些不同功能中的每一个。PCNA在其结构的同一区域(称为“域间连接环”)与不同的伴侣蛋白结合,但具有不同的亲和力。该域间连接环是固有的无序区域,当与不同的伴侣蛋白结合时,其具有不同的构象。在这项工作中,我们对未与任何伴侣蛋白结合的PCNA三聚体,与来自不同伴侣蛋白的肽结合的PCNA进行了全原子分子动力学模拟,PCNA和PCNA以两种不同的构象与完整的Fen 1蛋白结合。使用大量的模拟结果,我们分析了PCNA是否以其游离三聚体形式采样,其构象与结合至不同伴侣蛋白时的构象相似。我们观察到PCNA即使不与肽结合也对许多这些肽结合的构象进行采样,并且在与伴侣蛋白结合时选择特定的构象。我们还确定了在肽结合模拟中形成的PCNA-肽相互作用,在复合物的形成中起着至关重要的作用。计算的结合能与各种肽与PCNA的结合亲和力很好地相关。最后,我们研究了PCNA的内部动力学,并提出了PCNA招募具有约束力的合作伙伴的机制。
更新日期:2017-11-19
中文翻译:

增殖细胞核抗原如何调节与多个伴侣蛋白的结合特异性?
增殖细胞核抗原(PCNA)是滑动夹蛋白家族的成员,该蛋白在DNA修复,DNA复制,细胞周期控制和染色质修饰的多种形式中起着钳夹作用。PCNA充当同源三聚体,并与多种蛋白质复合,以执行这些不同功能中的每一个。PCNA在其结构的同一区域(称为“域间连接环”)与不同的伴侣蛋白结合,但具有不同的亲和力。该域间连接环是固有的无序区域,当与不同的伴侣蛋白结合时,其具有不同的构象。在这项工作中,我们对未与任何伴侣蛋白结合的PCNA三聚体,与来自不同伴侣蛋白的肽结合的PCNA进行了全原子分子动力学模拟,PCNA和PCNA以两种不同的构象与完整的Fen 1蛋白结合。使用大量的模拟结果,我们分析了PCNA是否以其游离三聚体形式采样,其构象与结合至不同伴侣蛋白时的构象相似。我们观察到PCNA即使不与肽结合也对许多这些肽结合的构象进行采样,并且在与伴侣蛋白结合时选择特定的构象。我们还确定了在肽结合模拟中形成的PCNA-肽相互作用,在复合物的形成中起着至关重要的作用。计算的结合能与各种肽与PCNA的结合亲和力很好地相关。最后,我们研究了PCNA的内部动力学,并提出了PCNA招募具有约束力的合作伙伴的机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号