Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Size and Shape of Amyloid Fibrils Induced by Ganglioside Nanoclusters: Role of Sialyl Oligosaccharide in Fibril Formation
Langmuir ( IF 3.7 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.langmuir.7b02091 Teruhiko Matsubara 1 , Masaya Nishihara 1 , Hanaki Yasumori 1 , Mako Nakai 1 , Katsuhiko Yanagisawa 2 , Toshinori Sato 1
Langmuir ( IF 3.7 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.langmuir.7b02091 Teruhiko Matsubara 1 , Masaya Nishihara 1 , Hanaki Yasumori 1 , Mako Nakai 1 , Katsuhiko Yanagisawa 2 , Toshinori Sato 1
Affiliation
|
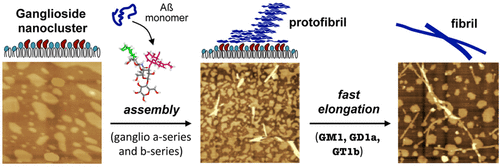
Ganglioside-enriched microdomains in the presynaptic neuronal membrane play a key role in the initiation of amyloid ß-protein (Aß) assembly related to Alzheimer’s disease. We previously isolated lipids from a detergent-resistant membrane microdomain fraction of synaptosomes prepared from aged mouse brain and found that spherical Aß assemblies were formed on Aß-sensitive ganglioside nanoclusters (ASIGN) of reconstituted lipid bilayers in the synaptosomal fraction. In the present study, we investigated the role of oligosaccharides in Aß fibril formation induced by ganglioside-containing mixed lipid membranes that mimic the features of ASIGN. Ganglioside nanoclusters were constructed as ternary mixed lipid bilayers composed of ganglioside (GM1, GM2, GM3, GD1a, or GT1b), sphingomyelin, and cholesterol, and their surface topography was visualized by atomic force microscopy. Aß fibril formation on the nanocluster was strongly induced in the presence of 10 mol % ganglioside, and Aß-sensitive features were observed at cholesterol contents of 35–55 mol %. GM1-, GD1a-, and GT1b-containing membranes induced longer fibrils than those containing GD1b and GM2, indicating that the terminal galactose of GM1 along with N-acetylneuraminic acid accelerates protofibril elongation. These results demonstrate that Aß fibril formation is induced by ASIGN that are highly enriched ganglioside nanoclusters with a limited number of components and that the generation and elongation of Aß protofibrils are regulated by the oligosaccharide structure of gangliosides.
中文翻译:

神经节苷脂纳米簇诱导淀粉样蛋白原纤维的大小和形状:唾液酸寡糖在原纤维形成中的作用。
突触前神经元膜中富含神经节苷脂的微区在与阿尔茨海默氏病有关的淀粉样β蛋白(Aβ)组装的启动中起关键作用。我们以前从老年小鼠大脑制备的突触体的耐去污剂的膜微区级分中分离了脂质,发现在突触体级分中重组脂质双层的Aß敏感神经节苷脂纳米簇(ASIGN)上形成了球形Aß装配体。在本研究中,我们调查了寡糖在由模拟ASIGN特征的含神经节苷脂的混合脂质膜诱导的Aß原纤维形成中的作用。神经节苷脂纳米簇被构建为由神经节苷脂(GM1,GM2,GM3,GD1a或GT1b),鞘磷脂和胆固醇组成的三元混合脂质双层。并通过原子力显微镜观察其表面形貌。在神经节苷脂含量为10 mol%的情况下,强烈诱导了纳米簇上的Aß原纤维形成,并且在胆固醇含量为35–55 mol%时观察到了Aß敏感特征。包含GM1,GD1a和GT1b的膜比包含GD1b和GM2的膜诱导的原纤维更长,表明GM1的末端半乳糖与N-乙酰神经氨酸加速原纤维的伸长。这些结果表明,ASIGN可以诱导Aß原纤维的形成,ASIGN是高度富集的神经节苷脂纳米簇,具有有限数量的组分,并且Aβ原纤维的生成和伸长受神经节苷脂的寡糖结构调控。
更新日期:2017-11-17
中文翻译:

神经节苷脂纳米簇诱导淀粉样蛋白原纤维的大小和形状:唾液酸寡糖在原纤维形成中的作用。
突触前神经元膜中富含神经节苷脂的微区在与阿尔茨海默氏病有关的淀粉样β蛋白(Aβ)组装的启动中起关键作用。我们以前从老年小鼠大脑制备的突触体的耐去污剂的膜微区级分中分离了脂质,发现在突触体级分中重组脂质双层的Aß敏感神经节苷脂纳米簇(ASIGN)上形成了球形Aß装配体。在本研究中,我们调查了寡糖在由模拟ASIGN特征的含神经节苷脂的混合脂质膜诱导的Aß原纤维形成中的作用。神经节苷脂纳米簇被构建为由神经节苷脂(GM1,GM2,GM3,GD1a或GT1b),鞘磷脂和胆固醇组成的三元混合脂质双层。并通过原子力显微镜观察其表面形貌。在神经节苷脂含量为10 mol%的情况下,强烈诱导了纳米簇上的Aß原纤维形成,并且在胆固醇含量为35–55 mol%时观察到了Aß敏感特征。包含GM1,GD1a和GT1b的膜比包含GD1b和GM2的膜诱导的原纤维更长,表明GM1的末端半乳糖与N-乙酰神经氨酸加速原纤维的伸长。这些结果表明,ASIGN可以诱导Aß原纤维的形成,ASIGN是高度富集的神经节苷脂纳米簇,具有有限数量的组分,并且Aβ原纤维的生成和伸长受神经节苷脂的寡糖结构调控。










































 京公网安备 11010802027423号
京公网安备 11010802027423号