当前位置:
X-MOL 学术
›
Soft Matter
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Van't Hoff's law for active suspensions: the role of the solvent chemical potential
Soft Matter ( IF 2.9 ) Pub Date : 2017-11-10 00:00:00 , DOI: 10.1039/c7sm01432e Jeroen Rodenburg 1, 2, 3, 4, 5 , Marjolein Dijkstra 3, 4, 5, 6, 7 , René van Roij 1, 2, 3, 4, 5
Soft Matter ( IF 2.9 ) Pub Date : 2017-11-10 00:00:00 , DOI: 10.1039/c7sm01432e Jeroen Rodenburg 1, 2, 3, 4, 5 , Marjolein Dijkstra 3, 4, 5, 6, 7 , René van Roij 1, 2, 3, 4, 5
Affiliation
|
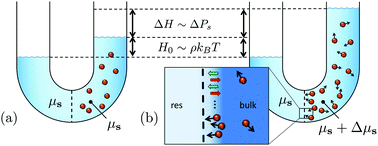
We extend Van’t Hoff's law for the osmotic pressure to a suspension of active Brownian particles. The propelled particles exert a net reaction force on the solvent, and thereby either drive a measurable solvent flow from the connecting solvent reservoir through the semipermeable membrane, or increase the osmotic pressure and cause the suspension to rise to heights as large as micrometers for experimentally realized microswimmers described in the literature. The increase in osmotic pressure is caused by the background solvent being, in contrast to passive suspensions, no longer at the chemical potential of the solvent reservoir. The difference in solvent chemical potentials depends on the colloid–membrane interaction potential, which implies that the osmotic pressure is a state function of a state that itself is influenced by the membrane potential.
中文翻译:

主动悬浮液的范霍夫定律:溶剂化学势的作用
我们将渗透压的范霍夫定律扩展到活性布朗粒子的悬浮液。被推进的颗粒对溶剂施加净反作用力,从而驱使可测量的溶剂从连接的溶剂容器中流过半透膜,或者增加渗透压并使悬浮液升至与实验实现的微米一样大的高度。文献中描述的微游泳者。渗透压的增加是由于与被动悬浮液不同,本底溶剂不再处于溶剂库的化学势。溶剂化学势的差异取决于胶体-膜相互作用的势,这意味着渗透压是其自身状态的状态函数 受膜电位的影响。
更新日期:2017-11-17
中文翻译:

主动悬浮液的范霍夫定律:溶剂化学势的作用
我们将渗透压的范霍夫定律扩展到活性布朗粒子的悬浮液。被推进的颗粒对溶剂施加净反作用力,从而驱使可测量的溶剂从连接的溶剂容器中流过半透膜,或者增加渗透压并使悬浮液升至与实验实现的微米一样大的高度。文献中描述的微游泳者。渗透压的增加是由于与被动悬浮液不同,本底溶剂不再处于溶剂库的化学势。溶剂化学势的差异取决于胶体-膜相互作用的势,这意味着渗透压是其自身状态的状态函数 受膜电位的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号