当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Toward Green Acylation of (Hetero)arenes: Palladium-Catalyzed Carbonylation of Olefins to Ketones
ACS Central Science ( IF 12.7 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1021/acscentsci.7b00368 Jie Liu 1 , Zhihong Wei 1 , Haijun Jiao 1 , Ralf Jackstell 1 , Matthias Beller 1
ACS Central Science ( IF 12.7 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1021/acscentsci.7b00368 Jie Liu 1 , Zhihong Wei 1 , Haijun Jiao 1 , Ralf Jackstell 1 , Matthias Beller 1
Affiliation
|
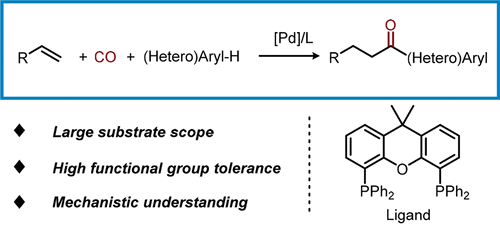
Green Friedel–Crafts acylation reactions belong to the most desired transformations in organic chemistry. The resulting ketones constitute important intermediates, building blocks, and functional molecules in organic synthesis as well as for the chemical industry. Over the past 60 years, advances in this topic have focused on how to make this reaction more economically and environmentally friendly by using green acylating conditions, such as stoichiometric acylations and catalytic homogeneous and heterogeneous acylations. However, currently well-established methodologies for their synthesis either produce significant amounts of waste or proceed under harsh conditions, limiting applications. Here, we present a new protocol for the straightforward and selective introduction of acyl groups into (hetero)arenes without directing groups by using available olefins with inexpensive CO. In the presence of commercial palladium catalysts, inter- and intramolecular carbonylative C–H functionalizations take place with good regio- and chemoselectivity. Compared to classical Friedel–Crafts chemistry, this novel methodology proceeds under mild reaction conditions. The general applicability of this methodology is demonstrated by the direct carbonylation of industrial feedstocks (ethylene and diisobutene) as well as of natural products (eugenol and safrole). Furthermore, synthetic applications to drug molecules are showcased.
中文翻译:

走向(杂)芳烃的绿色酰化:钯催化烯烃的羰基化成酮
Green Friedel-Crafts酰化反应属于有机化学中最需要的转变。所得酮构成有机合成以及化学工业中的重要中间体,结构单元和功能分子。在过去的60年中,该主题的进展集中在如何通过使用绿色的酰化条件(例如化学计量的酰化和催化的均相和异质的酰化)使该反应在经济上和环境上更加友好。但是,目前公认的合成方法学会产生大量的废物,或者在苛刻的条件下进行,从而限制了应用。这里,我们提出了一种新的协议,该协议通过使用便宜的CO和可用的烯烃,将酰基直接和选择性地引入(杂)芳烃中而没有直接的基团。在商业钯催化剂的存在下,分子间和分子内羰基化C–H官能化反应发生良好的区域选择性和化学选择性。与经典的Friedel-Crafts化学相比,这种新颖的方法在温和的反应条件下进行。该方法的一般适用性通过工业原料(乙烯和二异丁烯)以及天然产物(丁子香酚和丁香酚)的直接羰基化得到证明。此外,展示了对药物分子的合成应用。分子间和分子内羰基化C–H功能化具有良好的区域选择性和化学选择性。与经典的Friedel-Crafts化学相比,这种新颖的方法在温和的反应条件下进行。该方法的一般适用性通过工业原料(乙烯和二异丁烯)以及天然产物(丁子香酚和丁香酚)的直接羰基化得到证明。此外,展示了对药物分子的合成应用。分子间和分子内羰基化C–H功能化具有良好的区域选择性和化学选择性。与经典的Friedel-Crafts化学相比,这种新颖的方法在温和的反应条件下进行。该方法的一般适用性通过工业原料(乙烯和二异丁烯)以及天然产物(丁子香酚和丁香酚)的直接羰基化得到证明。此外,展示了对药物分子的合成应用。
更新日期:2017-11-16
中文翻译:

走向(杂)芳烃的绿色酰化:钯催化烯烃的羰基化成酮
Green Friedel-Crafts酰化反应属于有机化学中最需要的转变。所得酮构成有机合成以及化学工业中的重要中间体,结构单元和功能分子。在过去的60年中,该主题的进展集中在如何通过使用绿色的酰化条件(例如化学计量的酰化和催化的均相和异质的酰化)使该反应在经济上和环境上更加友好。但是,目前公认的合成方法学会产生大量的废物,或者在苛刻的条件下进行,从而限制了应用。这里,我们提出了一种新的协议,该协议通过使用便宜的CO和可用的烯烃,将酰基直接和选择性地引入(杂)芳烃中而没有直接的基团。在商业钯催化剂的存在下,分子间和分子内羰基化C–H官能化反应发生良好的区域选择性和化学选择性。与经典的Friedel-Crafts化学相比,这种新颖的方法在温和的反应条件下进行。该方法的一般适用性通过工业原料(乙烯和二异丁烯)以及天然产物(丁子香酚和丁香酚)的直接羰基化得到证明。此外,展示了对药物分子的合成应用。分子间和分子内羰基化C–H功能化具有良好的区域选择性和化学选择性。与经典的Friedel-Crafts化学相比,这种新颖的方法在温和的反应条件下进行。该方法的一般适用性通过工业原料(乙烯和二异丁烯)以及天然产物(丁子香酚和丁香酚)的直接羰基化得到证明。此外,展示了对药物分子的合成应用。分子间和分子内羰基化C–H功能化具有良好的区域选择性和化学选择性。与经典的Friedel-Crafts化学相比,这种新颖的方法在温和的反应条件下进行。该方法的一般适用性通过工业原料(乙烯和二异丁烯)以及天然产物(丁子香酚和丁香酚)的直接羰基化得到证明。此外,展示了对药物分子的合成应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号