当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An exploration into the amide–pseudo amide hydrogen bonding synthon between a new coformer with two primary amide groups and theophylline
CrystEngComm ( IF 2.6 ) Pub Date : 2017-11-06 00:00:00 , DOI: 10.1039/c7ce01666b Datta Markad 1, 2, 3, 4 , Sanjay K. Mandal 1, 2, 3, 4
CrystEngComm ( IF 2.6 ) Pub Date : 2017-11-06 00:00:00 , DOI: 10.1039/c7ce01666b Datta Markad 1, 2, 3, 4 , Sanjay K. Mandal 1, 2, 3, 4
Affiliation
|
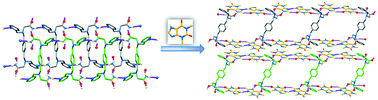
A cocrystal between a new coformer with two primary amide groups, 2,2′-((1,4-phenylenebis(methylene))-bis((pyridin-2-ylmethyl)azanediyl))diacetamide (2-BPXG), and theophylline (THP) was selected as a model system to (a) demonstrate the presence of a rare amide–pseudo amide hydrogen bonding synthon in it and identify further the structural features by single crystal X-ray diffraction, and (b) establish its relevant physicochemical properties through a comparison with the coformer by Fourier-transform infrared (FT-IR) spectroscopy, differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), differential thermal analysis (DTA), powder X-ray diffraction (PXRD), and hot stage microscopy (HSM). To the best of our knowledge, this is the first example where a coformer with two primary amide groups has been used to form the amide–pseudo amide hydrogen bonding synthon. The co-crystal (2-BPXG·4THP) crystallizes in the triclinic space group P![[1 with combining macron]](http://www.rsc.org/images/entities/char_0031_0304.gif) with Z = 1, where the unit cell contains one 2-BPXG molecule and four THP molecules. Meanwhile, the coformer 2-BPXG crystallizes in the monoclinic space group P21/c with Z = 2 (the asymmetric unit contains half of the molecule). Surprisingly, only 2-BPXG (compared to other coformers with aliphatic spacers between the two alkyl nitrogen atoms), which does not form the amide–amide hydrogen bonding synthon within itself, paves the way for the formation of the amide–pseudo amide hydrogen bonding synthon R22(9) with THP. An overall 2D supramolecular network is formed in 2-BPXG through the interlinking of ladder-shaped layers (which are generated through strong hydrogen bonding between one of the N–H bonds and pyridine nitrogen) via strong hydrogen bonding between the other N–H bond and the carbonyl group of an adjacent molecule. On the other hand, the coformer with one primary amide group on each end generates a ladder-shaped layer in the cocrystal through hydrogen bonding interactions with THP molecules. These ladder-shaped layers are further connected via strong π–π (centroid to centroid distance: 3.68 Å) and weak C–H⋯O interactions between the THP molecules to form an overall 3D supramolecular network in the cocrystal. Hydrogen bond propensities, Hirshfeld surface analysis and quantitative crystal structure analysis of both coformer and cocrystal allowed us to understand the amide–pseudo amide hydrogen bonding synthon in detail.
with Z = 1, where the unit cell contains one 2-BPXG molecule and four THP molecules. Meanwhile, the coformer 2-BPXG crystallizes in the monoclinic space group P21/c with Z = 2 (the asymmetric unit contains half of the molecule). Surprisingly, only 2-BPXG (compared to other coformers with aliphatic spacers between the two alkyl nitrogen atoms), which does not form the amide–amide hydrogen bonding synthon within itself, paves the way for the formation of the amide–pseudo amide hydrogen bonding synthon R22(9) with THP. An overall 2D supramolecular network is formed in 2-BPXG through the interlinking of ladder-shaped layers (which are generated through strong hydrogen bonding between one of the N–H bonds and pyridine nitrogen) via strong hydrogen bonding between the other N–H bond and the carbonyl group of an adjacent molecule. On the other hand, the coformer with one primary amide group on each end generates a ladder-shaped layer in the cocrystal through hydrogen bonding interactions with THP molecules. These ladder-shaped layers are further connected via strong π–π (centroid to centroid distance: 3.68 Å) and weak C–H⋯O interactions between the THP molecules to form an overall 3D supramolecular network in the cocrystal. Hydrogen bond propensities, Hirshfeld surface analysis and quantitative crystal structure analysis of both coformer and cocrystal allowed us to understand the amide–pseudo amide hydrogen bonding synthon in detail.
中文翻译:

具有两个伯酰胺基团的新型共形成剂与茶碱之间的酰胺-拟酰胺氢键合成子的探索
具有两个伯酰胺基团的新型共形成剂,2,2'-(((1,4-亚苯基双(亚甲基))-双((吡啶-2-基甲基)氮杂二基))二乙酰胺(2-BPXG)与茶碱的共晶体(THP)被选作模型系统,以(a)证明其中存在稀有酰胺-拟酰胺氢键合成子,并通过单晶X射线衍射进一步鉴定结构特征,以及(b)通过建立其相关的理化性质通过傅立叶变换红外(FT-IR)光谱,差示扫描量热法(DSC),热重分析(TGA),差示热分析(DTA),粉末X射线衍射(PXRD)和热台显微镜与共形成物进行比较(HSM)。据我们所知,这是第一个使用具有两个伯酰胺基团的共形成物形成酰胺-假酰胺氢键合成子的例子。共晶(2-BPXG·4THP)在三斜晶空间群P中结晶![[1个结合宏]](http://www.rsc.org/images/entities/char_0031_0304.gif) ,Z = 1,其中晶胞包含一个2-BPXG分子和四个THP分子。同时,共形成2- BPXG结晶的单斜晶系空间群P 2 1 / ç与ž = 2(不对称单元包含分子的一半)。出乎意料的是,只有2-BPXG(与在两个烷基氮原子之间带有脂肪族间隔基的其他共聚体相比)本身并不形成酰胺-酰胺氢键合成子,这为酰胺-拟酰胺氢键的形成铺平了道路。具有THP的synthon R 2 2(9)。形成为整体的二维超分子网络2- BPXG通过的(其通过强的氢键的N-H键和吡啶氮中的一个之间产生的)梯子状层中的互连通过其他N-H键之间的强氢键和相邻分子的羰基。另一方面,在每个末端具有一个伯酰胺基团的共形成物通过与THP分子的氢键相互作用在共晶体中生成梯形层。这些梯形层通过强大的π–π(质心到质心距离:3.68Å)和THP之间的弱C–H⋯O相互作用进一步连接分子在共晶中形成整体3D超分子网络。氢键的性质,Hirshfeld表面分析以及共形成物和共晶体的定量晶体结构分析使我们能够详细了解酰胺-伪酰胺氢键合成子。
,Z = 1,其中晶胞包含一个2-BPXG分子和四个THP分子。同时,共形成2- BPXG结晶的单斜晶系空间群P 2 1 / ç与ž = 2(不对称单元包含分子的一半)。出乎意料的是,只有2-BPXG(与在两个烷基氮原子之间带有脂肪族间隔基的其他共聚体相比)本身并不形成酰胺-酰胺氢键合成子,这为酰胺-拟酰胺氢键的形成铺平了道路。具有THP的synthon R 2 2(9)。形成为整体的二维超分子网络2- BPXG通过的(其通过强的氢键的N-H键和吡啶氮中的一个之间产生的)梯子状层中的互连通过其他N-H键之间的强氢键和相邻分子的羰基。另一方面,在每个末端具有一个伯酰胺基团的共形成物通过与THP分子的氢键相互作用在共晶体中生成梯形层。这些梯形层通过强大的π–π(质心到质心距离:3.68Å)和THP之间的弱C–H⋯O相互作用进一步连接分子在共晶中形成整体3D超分子网络。氢键的性质,Hirshfeld表面分析以及共形成物和共晶体的定量晶体结构分析使我们能够详细了解酰胺-伪酰胺氢键合成子。
更新日期:2017-11-17
![[1 with combining macron]](http://www.rsc.org/images/entities/char_0031_0304.gif) with Z = 1, where the unit cell contains one 2-BPXG molecule and four THP molecules. Meanwhile, the coformer 2-BPXG crystallizes in the monoclinic space group P21/c with Z = 2 (the asymmetric unit contains half of the molecule). Surprisingly, only 2-BPXG (compared to other coformers with aliphatic spacers between the two alkyl nitrogen atoms), which does not form the amide–amide hydrogen bonding synthon within itself, paves the way for the formation of the amide–pseudo amide hydrogen bonding synthon R22(9) with THP. An overall 2D supramolecular network is formed in 2-BPXG through the interlinking of ladder-shaped layers (which are generated through strong hydrogen bonding between one of the N–H bonds and pyridine nitrogen) via strong hydrogen bonding between the other N–H bond and the carbonyl group of an adjacent molecule. On the other hand, the coformer with one primary amide group on each end generates a ladder-shaped layer in the cocrystal through hydrogen bonding interactions with THP molecules. These ladder-shaped layers are further connected via strong π–π (centroid to centroid distance: 3.68 Å) and weak C–H⋯O interactions between the THP molecules to form an overall 3D supramolecular network in the cocrystal. Hydrogen bond propensities, Hirshfeld surface analysis and quantitative crystal structure analysis of both coformer and cocrystal allowed us to understand the amide–pseudo amide hydrogen bonding synthon in detail.
with Z = 1, where the unit cell contains one 2-BPXG molecule and four THP molecules. Meanwhile, the coformer 2-BPXG crystallizes in the monoclinic space group P21/c with Z = 2 (the asymmetric unit contains half of the molecule). Surprisingly, only 2-BPXG (compared to other coformers with aliphatic spacers between the two alkyl nitrogen atoms), which does not form the amide–amide hydrogen bonding synthon within itself, paves the way for the formation of the amide–pseudo amide hydrogen bonding synthon R22(9) with THP. An overall 2D supramolecular network is formed in 2-BPXG through the interlinking of ladder-shaped layers (which are generated through strong hydrogen bonding between one of the N–H bonds and pyridine nitrogen) via strong hydrogen bonding between the other N–H bond and the carbonyl group of an adjacent molecule. On the other hand, the coformer with one primary amide group on each end generates a ladder-shaped layer in the cocrystal through hydrogen bonding interactions with THP molecules. These ladder-shaped layers are further connected via strong π–π (centroid to centroid distance: 3.68 Å) and weak C–H⋯O interactions between the THP molecules to form an overall 3D supramolecular network in the cocrystal. Hydrogen bond propensities, Hirshfeld surface analysis and quantitative crystal structure analysis of both coformer and cocrystal allowed us to understand the amide–pseudo amide hydrogen bonding synthon in detail.
中文翻译:

具有两个伯酰胺基团的新型共形成剂与茶碱之间的酰胺-拟酰胺氢键合成子的探索
具有两个伯酰胺基团的新型共形成剂,2,2'-(((1,4-亚苯基双(亚甲基))-双((吡啶-2-基甲基)氮杂二基))二乙酰胺(2-BPXG)与茶碱的共晶体(THP)被选作模型系统,以(a)证明其中存在稀有酰胺-拟酰胺氢键合成子,并通过单晶X射线衍射进一步鉴定结构特征,以及(b)通过建立其相关的理化性质通过傅立叶变换红外(FT-IR)光谱,差示扫描量热法(DSC),热重分析(TGA),差示热分析(DTA),粉末X射线衍射(PXRD)和热台显微镜与共形成物进行比较(HSM)。据我们所知,这是第一个使用具有两个伯酰胺基团的共形成物形成酰胺-假酰胺氢键合成子的例子。共晶(2-BPXG·4THP)在三斜晶空间群P中结晶
![[1个结合宏]](http://www.rsc.org/images/entities/char_0031_0304.gif) ,Z = 1,其中晶胞包含一个2-BPXG分子和四个THP分子。同时,共形成2- BPXG结晶的单斜晶系空间群P 2 1 / ç与ž = 2(不对称单元包含分子的一半)。出乎意料的是,只有2-BPXG(与在两个烷基氮原子之间带有脂肪族间隔基的其他共聚体相比)本身并不形成酰胺-酰胺氢键合成子,这为酰胺-拟酰胺氢键的形成铺平了道路。具有THP的synthon R 2 2(9)。形成为整体的二维超分子网络2- BPXG通过的(其通过强的氢键的N-H键和吡啶氮中的一个之间产生的)梯子状层中的互连通过其他N-H键之间的强氢键和相邻分子的羰基。另一方面,在每个末端具有一个伯酰胺基团的共形成物通过与THP分子的氢键相互作用在共晶体中生成梯形层。这些梯形层通过强大的π–π(质心到质心距离:3.68Å)和THP之间的弱C–H⋯O相互作用进一步连接分子在共晶中形成整体3D超分子网络。氢键的性质,Hirshfeld表面分析以及共形成物和共晶体的定量晶体结构分析使我们能够详细了解酰胺-伪酰胺氢键合成子。
,Z = 1,其中晶胞包含一个2-BPXG分子和四个THP分子。同时,共形成2- BPXG结晶的单斜晶系空间群P 2 1 / ç与ž = 2(不对称单元包含分子的一半)。出乎意料的是,只有2-BPXG(与在两个烷基氮原子之间带有脂肪族间隔基的其他共聚体相比)本身并不形成酰胺-酰胺氢键合成子,这为酰胺-拟酰胺氢键的形成铺平了道路。具有THP的synthon R 2 2(9)。形成为整体的二维超分子网络2- BPXG通过的(其通过强的氢键的N-H键和吡啶氮中的一个之间产生的)梯子状层中的互连通过其他N-H键之间的强氢键和相邻分子的羰基。另一方面,在每个末端具有一个伯酰胺基团的共形成物通过与THP分子的氢键相互作用在共晶体中生成梯形层。这些梯形层通过强大的π–π(质心到质心距离:3.68Å)和THP之间的弱C–H⋯O相互作用进一步连接分子在共晶中形成整体3D超分子网络。氢键的性质,Hirshfeld表面分析以及共形成物和共晶体的定量晶体结构分析使我们能够详细了解酰胺-伪酰胺氢键合成子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号