当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Polyradical PROXYL/TEMPO-Derived Amides: Synthesis, Physicochemical Studies, DFT Calculations, and Antimicrobial Activity
ChemPlusChem ( IF 3.0 ) Pub Date : 2017-11-17 04:40:54 , DOI: 10.1002/cplu.201700343 Patrik Poprac 1 , Peter Poliak 1 , Miroslav Kavala 1 , Zuzana Barbieriková 1 , Michal Zalibera 1 , Marek Fronc 1 , Ľubomír Švorc 1 , Zuzana Vihonská 1 , Petra Olejníková 1 , Karol Lušpai 1 , Vladimír Lukeš 1 , Vlasta Brezová 1 , Peter Szolcsányi 1
ChemPlusChem ( IF 3.0 ) Pub Date : 2017-11-17 04:40:54 , DOI: 10.1002/cplu.201700343 Patrik Poprac 1 , Peter Poliak 1 , Miroslav Kavala 1 , Zuzana Barbieriková 1 , Michal Zalibera 1 , Marek Fronc 1 , Ľubomír Švorc 1 , Zuzana Vihonská 1 , Petra Olejníková 1 , Karol Lušpai 1 , Vladimír Lukeš 1 , Vlasta Brezová 1 , Peter Szolcsányi 1
Affiliation
|
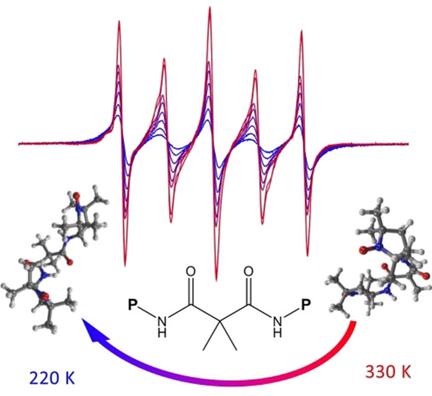
Flexibility and communication: A series of di- and polynitroxide amides possessing 2,2,5,5-tetramethyl-1-pyrrolidinyloxy (PROXYL) and/or 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) units were synthesized and their properties were analyzed through multiple experimental techniques, including X- and Q-band EPR spectroscopy, X-ray diffraction, cyclic voltammetry, and DFT calculations (see figure). An assessment of their inhibitory activities against selected bacteria, yeasts, and filamentous fungi revealed the highest activity for TEMPO-derived tetraradicals.
中文翻译:

聚自由基PROXYL / TEMPO衍生的酰胺:合成,物化研究,DFT计算和抗菌活性
灵活性和沟通性:一系列具有2,2,5,5-四甲基-1-吡咯烷氧基(PROXYL)和/或2,2,6,6-四甲基-1-哌啶基氧基(TEMPO)的二硝基和聚硝基酰胺是通过多种实验技术,包括X波段和Q波段EPR光谱,X射线衍射,循环伏安法和DFT计算,分析了它们的合成及其性质。对它们对所选细菌,酵母和丝状真菌的抑制活性的评估显示,TEMPO衍生的四基自由基的活性最高。
更新日期:2017-11-17
中文翻译:

聚自由基PROXYL / TEMPO衍生的酰胺:合成,物化研究,DFT计算和抗菌活性
灵活性和沟通性:一系列具有2,2,5,5-四甲基-1-吡咯烷氧基(PROXYL)和/或2,2,6,6-四甲基-1-哌啶基氧基(TEMPO)的二硝基和聚硝基酰胺是通过多种实验技术,包括X波段和Q波段EPR光谱,X射线衍射,循环伏安法和DFT计算,分析了它们的合成及其性质。对它们对所选细菌,酵母和丝状真菌的抑制活性的评估显示,TEMPO衍生的四基自由基的活性最高。











































 京公网安备 11010802027423号
京公网安备 11010802027423号