当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bicarbonate Is Not a General Acid in Au-Catalyzed CO2 Electroreduction
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1021/jacs.7b08345 Anna Wuttig 1 , Youngmin Yoon 1 , Jaeyune Ryu 1 , Yogesh Surendranath 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1021/jacs.7b08345 Anna Wuttig 1 , Youngmin Yoon 1 , Jaeyune Ryu 1 , Yogesh Surendranath 1
Affiliation
|
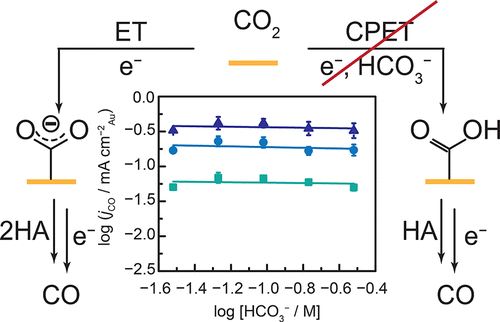
We show that bicarbonate is neither a general acid nor a reaction partner in the rate-limiting step of electrochemical CO2 reduction catalysis mediated by planar polycrystalline Au surfaces. We formulate microkinetic models and propose diagnostic criteria to distinguish the role of bicarbonate. Comparing these models with the observed zero-order dependence in bicarbonate and simulated interfacial concentration gradients, we conclude that bicarbonate is not a general acid cocatalyst. Instead, it acts as a viable proton donor past the rate-limiting step and a sluggish buffer that maintains the bulk but not local pH in CO2-saturated aqueous electrolytes.
中文翻译:

碳酸氢盐不是Au催化的CO 2电还原中的通用酸
我们显示碳酸氢盐既不是一般的酸,也不是平面多晶金表面介导的电化学CO 2还原催化的限速步骤中的反应伙伴。我们制定微观动力学模型,并提出诊断标准,以区分碳酸氢盐的作用。将这些模型与在碳酸氢盐和模拟的界面浓度梯度中观察到的零级依赖性进行比较,我们得出的结论是,碳酸氢盐不是一般的酸助催化剂。相反,它在限速步骤之后充当可行的质子供体,并且是缓慢的缓冲剂,该缓冲剂在CO 2饱和的水性电解质中保持整体而不是局部pH值。
更新日期:2017-11-17
中文翻译:

碳酸氢盐不是Au催化的CO 2电还原中的通用酸
我们显示碳酸氢盐既不是一般的酸,也不是平面多晶金表面介导的电化学CO 2还原催化的限速步骤中的反应伙伴。我们制定微观动力学模型,并提出诊断标准,以区分碳酸氢盐的作用。将这些模型与在碳酸氢盐和模拟的界面浓度梯度中观察到的零级依赖性进行比较,我们得出的结论是,碳酸氢盐不是一般的酸助催化剂。相反,它在限速步骤之后充当可行的质子供体,并且是缓慢的缓冲剂,该缓冲剂在CO 2饱和的水性电解质中保持整体而不是局部pH值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号