当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tandem [1,2]‐Wittig Rearrangement/Lactonization of γ‐Benzyloxy Vinylogous Urethanes: Application to the Synthetic Studies of Maculalactone A, Planchol C and γ‐Lycorane
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-11-30 , DOI: 10.1002/ajoc.201700589 Guo-Ming Ho,Yu-Jang Li
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-11-30 , DOI: 10.1002/ajoc.201700589 Guo-Ming Ho,Yu-Jang Li
|
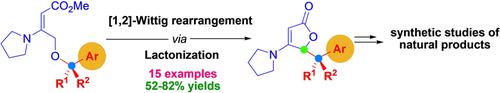
A tandem [1,2]‐Wittig rearrangement/lactonization of γ‐benzyloxy vinylogous urethanes is presented herein. The resulting butenolides featuring various benzyl substituents at the γ‐position are structurally diverse compounds found in natural products. The usefulness of this synthetic approach was demonstrated in the formal synthesis of maculalactone A, rapid assembly of the tricyclic core of planchol C, and the total synthesis of γ‐lycorane.
中文翻译:

串联[1,2] -Wittig重排/内酯化γ-苄氧基乙烯基氨基甲酸酯:在黄酮内酯A,Planchol C和γ-Lycorane合成研究中的应用
本文介绍了γ-苄氧基乙烯基氨基甲酸酯的串联[1,2] -Wittig重排/内酯化。所得的丁烯内酯在γ位具有各种苄基取代基,是天然产物中结构上不同的化合物。这种合成方法的有效性在黄酮内酯A的正式合成,戊二酚C的三环核的快速组装以及γ-二十烷的总合成中得到了证明。
更新日期:2017-11-30
中文翻译:

串联[1,2] -Wittig重排/内酯化γ-苄氧基乙烯基氨基甲酸酯:在黄酮内酯A,Planchol C和γ-Lycorane合成研究中的应用
本文介绍了γ-苄氧基乙烯基氨基甲酸酯的串联[1,2] -Wittig重排/内酯化。所得的丁烯内酯在γ位具有各种苄基取代基,是天然产物中结构上不同的化合物。这种合成方法的有效性在黄酮内酯A的正式合成,戊二酚C的三环核的快速组装以及γ-二十烷的总合成中得到了证明。











































 京公网安备 11010802027423号
京公网安备 11010802027423号