当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereo- and Regioselective Addition of Arene to Alkyne Using Abnormal NHC Based Palladium Catalysts: Elucidating the Role of Trifluoroacetic Acid in Fujiwara Process
Organometallics ( IF 2.5 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acs.organomet.7b00649 Pradip Kumar Hota 1 , Anex Jose 1 , Swadhin K. Mandal 1
Organometallics ( IF 2.5 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acs.organomet.7b00649 Pradip Kumar Hota 1 , Anex Jose 1 , Swadhin K. Mandal 1
Affiliation
|
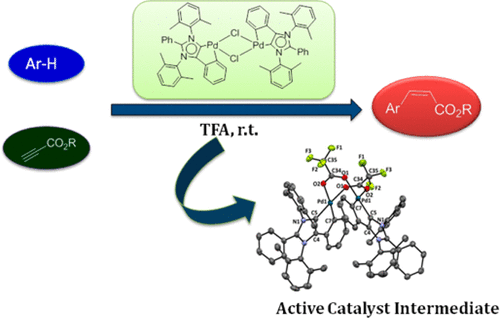
Hydroarylation of alkyne was reported by Fujiwara nearly two decades back. Interestingly, this reaction does not proceed in the absence of trifluoroacetic acid; however, the exact role of TFA has not been unambiguously established, in particular with the support of X-ray crystallography by isolating the TFA-involved catalytically active species. In this work, abnormal N-heterocyclic carbene (aNHC) based Pd catalysts have been used for the efficient hydroarylation of aromatic C–H bonds leading to new C–C bond formation through regio- and stereoselective addition to alkynes. The addition reaction has been realized by a catalytic amount of Pd (II) compound (0.5 mol %) in trifluoroacetic acid (TFA) under ambient conditions. Various arenes undergo transhydroarylation selectively across the triple bond (containing functional groups CO2Me, CO2Et, and CO2H), affording the kinetically controlled cis adduct predominantly in good yields. A simple reaction condition through an intermolecular reaction has been outlined under ambient conditions for the synthesis of coumarin derivatives, which are considered as an important class of bioactive compounds. It was noted that the reaction does not proceed in the absence of TFA. Hence, the major emphasis was given to understand the role of TFA in such hydroarylation reactions. A catalytically active reaction intermediate, [aNHCPd(CF3COO)]2, containing trifluoroacetate anion was characterized as the first solid-state evidence by single-crystal X-ray crystallography, which helps to understand the exact role of TFA in such a reaction. A detailed mechanistic understanding of this fascinating catalytic process has been proposed by tandem experimental and computational experiments.
中文翻译:

使用异常的基于NHC的钯催化剂将芳烃立体和区域选择性加成到炔烃上:阐明三氟乙酸在藤原工艺中的作用
近二十年前的藤原报道了炔烃的加氢芳基化反应。有趣的是,在不存在三氟乙酸的情况下该反应不会进行。然而,TFA的确切作用尚未明确确定,特别是在X射线晶体学的支持下,通过分离涉及TFA的催化活性物质。在这项工作中,异常N-杂环卡宾(一基于NHC)的Pd催化剂已用于芳族C-H键的有效加氢芳基化,从而通过向炔烃中进行区域和立体选择性加成而形成新的C-C键。通过在环境条件下在三氟乙酸(TFA)中催化量的Pd(II)化合物(0.5mol%)实现了加成反应。各种芳烃选择性地通过三键(包含官能团CO 2 Me,CO 2 Et和CO 2H),主要以高收率得到动力学控制的顺式加合物。已经概述了在环境条件下通过分子间反应的简单反应条件来合成香豆素衍生物,香豆素衍生物被认为是一类重要的生物活性化合物。注意到在没有TFA的情况下反应不会进行。因此,主要重点是了解TFA在此类氢芳基化反应中的作用。甲催化活性反应中间体,[一NHCPd(CF 3 COO)] 2含有三氟乙酸根阴离子的单晶X射线晶体学表征其为第一批固态证据,这有助于了解TFA在此类反应中的确切作用。通过串联实验和计算实验已经提出了对该迷人的催化过程的详细机械理解。
更新日期:2017-11-16
中文翻译:

使用异常的基于NHC的钯催化剂将芳烃立体和区域选择性加成到炔烃上:阐明三氟乙酸在藤原工艺中的作用
近二十年前的藤原报道了炔烃的加氢芳基化反应。有趣的是,在不存在三氟乙酸的情况下该反应不会进行。然而,TFA的确切作用尚未明确确定,特别是在X射线晶体学的支持下,通过分离涉及TFA的催化活性物质。在这项工作中,异常N-杂环卡宾(一基于NHC)的Pd催化剂已用于芳族C-H键的有效加氢芳基化,从而通过向炔烃中进行区域和立体选择性加成而形成新的C-C键。通过在环境条件下在三氟乙酸(TFA)中催化量的Pd(II)化合物(0.5mol%)实现了加成反应。各种芳烃选择性地通过三键(包含官能团CO 2 Me,CO 2 Et和CO 2H),主要以高收率得到动力学控制的顺式加合物。已经概述了在环境条件下通过分子间反应的简单反应条件来合成香豆素衍生物,香豆素衍生物被认为是一类重要的生物活性化合物。注意到在没有TFA的情况下反应不会进行。因此,主要重点是了解TFA在此类氢芳基化反应中的作用。甲催化活性反应中间体,[一NHCPd(CF 3 COO)] 2含有三氟乙酸根阴离子的单晶X射线晶体学表征其为第一批固态证据,这有助于了解TFA在此类反应中的确切作用。通过串联实验和计算实验已经提出了对该迷人的催化过程的详细机械理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号