当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Reactivity of Model Intermediates Proposed for the Pd-Catalyzed Remote C–H Functionalization of N-(2-Haloaryl)acrylamides
Organometallics ( IF 2.5 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acs.organomet.7b00702 Marta Pérez-Gómez 1 , Leticia Navarro 1 , Isabel Saura-Llamas 1 , Delia Bautista 2 , Mark Lautens 3 , José-Antonio García-López 1
Organometallics ( IF 2.5 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acs.organomet.7b00702 Marta Pérez-Gómez 1 , Leticia Navarro 1 , Isabel Saura-Llamas 1 , Delia Bautista 2 , Mark Lautens 3 , José-Antonio García-López 1
Affiliation
|
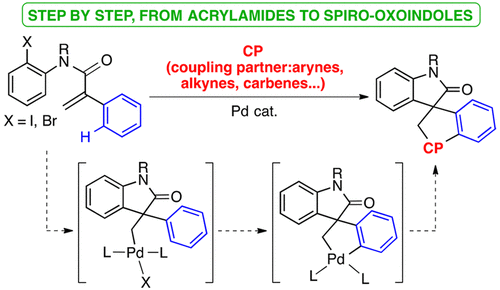
We have studied the possible reaction pathways operating in the Pd-catalyzed remote C–H functionalization of N-(2-haloaryl)acrylamides from an organometallic approach. We have isolated and characterized several proposed reaction intermediates, such as σ-alkyl-Pd complexes and spiro C,C-palladacycles, and evaluated the role of the base and the auxiliary ligands coordinated to Pd in the remote C–H activation process. In addition, the reactivity of these intermediates toward different unsaturated species such as benzyne, alkynes, and isocyanides has been studied in order to gain further insight into the reaction mechanism leading to functionalized spiro-oxoindoles.
中文翻译:

N-(2-卤代芳基)丙烯酰胺的Pd催化远程C–H官能化的模型中间体的合成和反应性
我们已经研究了有机金属方法中N-(2-卤代芳基)丙烯酰胺在Pd催化的远程C–H官能化中的可能反应途径。我们已经分离并表征了几种拟议的反应中间体,例如σ-烷基-Pd配合物和螺C,C-钯环,并评估了碱和与Pd配位的辅助配体在远程C–H活化过程中的作用。另外,已经研究了这些中间体对不同的不饱和物种,例如苯并,炔和异氰酸酯的反应性,以进一步了解导致官能化螺-氧吲哚的反应机理。
更新日期:2017-11-16
中文翻译:

N-(2-卤代芳基)丙烯酰胺的Pd催化远程C–H官能化的模型中间体的合成和反应性
我们已经研究了有机金属方法中N-(2-卤代芳基)丙烯酰胺在Pd催化的远程C–H官能化中的可能反应途径。我们已经分离并表征了几种拟议的反应中间体,例如σ-烷基-Pd配合物和螺C,C-钯环,并评估了碱和与Pd配位的辅助配体在远程C–H活化过程中的作用。另外,已经研究了这些中间体对不同的不饱和物种,例如苯并,炔和异氰酸酯的反应性,以进一步了解导致官能化螺-氧吲哚的反应机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号