当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unconventional Method for Synthesis of 3-Carboxyethyl-4-formyl(hydroxy)-5-aryl-N-arylpyrazoles
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acs.joc.7b02361 Michael J. V. da Silva 1 , Julia Poletto 1 , Andrey P. Jacomini 1 , Karlos E. Pianoski 1 , Davana S. Gonçalves 1 , Gessica M. Ribeiro 1 , Samara M. de S. Melo 1 , Davi F. Back 2 , Sidnei Moura 3 , Fernanda A. Rosa 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acs.joc.7b02361 Michael J. V. da Silva 1 , Julia Poletto 1 , Andrey P. Jacomini 1 , Karlos E. Pianoski 1 , Davana S. Gonçalves 1 , Gessica M. Ribeiro 1 , Samara M. de S. Melo 1 , Davi F. Back 2 , Sidnei Moura 3 , Fernanda A. Rosa 1
Affiliation
|
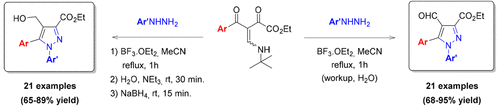
An alternative highly regioselective synthetic method for the preparation of 3,5-disubstituted 4-formyl-N-arylpyrazoles in a one-pot procedure is reported. The methodology developed was based on the regiochemical control of the cyclocondensation reaction of β-enamino diketones with arylhydrazines. Structural modifications in the β-enamino diketone system allied to the Lewis acid carbonyl activator BF3 were strategically employed for this control. Also a one-pot method for the preparation of 3,5-disubstituted 4-hydroxymethyl-N-arylpyrazole derivatives from the β-enamino diketone and arylhydrazine substrates is described.
中文翻译:

非传统方法合成3-羧乙基-4-甲酰基(羟基)-5-芳基-N-芳基吡唑
报道了一种通过一锅法制备3,5-二取代的4-甲酰基-N-芳基吡唑类化合物的另一种高度区域选择性的合成方法。所开发的方法基于对β-烯氨基二酮与芳基肼的环缩合反应的区域化学控制。与路易斯酸羰基活化剂BF 3相关联的β-烯氨基二酮系统的结构修饰被策略性地用于该控制。还描述了一种由一锅法从β-烯氨基二酮和芳基肼底物制备3,5-二取代的4-羟甲基-N-芳基吡唑衍生物的方法。
更新日期:2017-11-16
中文翻译:

非传统方法合成3-羧乙基-4-甲酰基(羟基)-5-芳基-N-芳基吡唑
报道了一种通过一锅法制备3,5-二取代的4-甲酰基-N-芳基吡唑类化合物的另一种高度区域选择性的合成方法。所开发的方法基于对β-烯氨基二酮与芳基肼的环缩合反应的区域化学控制。与路易斯酸羰基活化剂BF 3相关联的β-烯氨基二酮系统的结构修饰被策略性地用于该控制。还描述了一种由一锅法从β-烯氨基二酮和芳基肼底物制备3,5-二取代的4-羟甲基-N-芳基吡唑衍生物的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号