当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rational Synthesis of 2-Bromoporphyrins and 2,12-Dibromoporphyrins
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acs.joc.7b02404 Anna-Bea Bornhof 1 , Ruisheng Xiong 1 , K. Eszter Borbas 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acs.joc.7b02404 Anna-Bea Bornhof 1 , Ruisheng Xiong 1 , K. Eszter Borbas 1
Affiliation
|
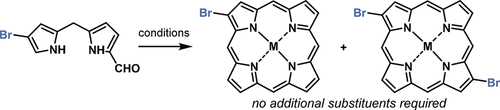
Bromoporphyrins were prepared by the metal-mediated self-condensation of brominated 1-formyldipyrromethanes. Depending on the conditions, Mg(II)-2,12-dibromoporphyrin and Mg(II)-2-bromoporphyrin could be obtained in up to 11% and 17% isolated yield, respectively. Zn(II) was also a viable templating metal. The positions of the bromine substituents were confirmed by 2D-NMR spectroscopic analysis and the X-ray crystal structure of a derivative. Suzuki and Sonogashira reactions of the bromoporphyrins yielded 2-substituted or 2,12-disubstituted porphyrins with red-shifted absorption and emission spectra. This method provides access to the minimalist core of β-mono- and β,β′-disubstituted porphyrins from readily available starting materials.
中文翻译:

2-溴卟啉和2,12-二溴卟啉的合理合成
溴卟啉是通过金属介导的溴化1-甲酰基二吡咯甲烷的自缩合反应制得的。根据条件,可以分别以高达11%和17%的分离产率获得Mg(II)-2,12-二溴卟啉和Mg(II)-2-溴卟啉。Zn(II)也是一种可行的模板金属。通过2D-NMR光谱分析和衍生物的X射线晶体结构确认了溴取代基的位置。溴卟啉的Suzuki和Sonogashira反应产生具有红移吸收和发射光谱的2-取代或2,12-二取代的卟啉。该方法提供了从容易获得的起始原料获得β-单-和β,β'-二取代的卟啉的极简核心的途径。
更新日期:2017-11-16
中文翻译:

2-溴卟啉和2,12-二溴卟啉的合理合成
溴卟啉是通过金属介导的溴化1-甲酰基二吡咯甲烷的自缩合反应制得的。根据条件,可以分别以高达11%和17%的分离产率获得Mg(II)-2,12-二溴卟啉和Mg(II)-2-溴卟啉。Zn(II)也是一种可行的模板金属。通过2D-NMR光谱分析和衍生物的X射线晶体结构确认了溴取代基的位置。溴卟啉的Suzuki和Sonogashira反应产生具有红移吸收和发射光谱的2-取代或2,12-二取代的卟啉。该方法提供了从容易获得的起始原料获得β-单-和β,β'-二取代的卟啉的极简核心的途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号