当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ring-Fused Cyclic Aminals from Tetrahydro-β-carboline-Based Dipeptide Compounds
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acs.joc.7b01656 Alessia Bertamino 1 , Gianluigi Lauro 1 , Carmine Ostacolo 2 , Veronica Di Sarno 1 , Simona Musella 1 , Tania Ciaglia 1 , Pietro Campiglia 1, 3 , Giuseppe Bifulco 1 , Isabel M. Gomez-Monterrey 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acs.joc.7b01656 Alessia Bertamino 1 , Gianluigi Lauro 1 , Carmine Ostacolo 2 , Veronica Di Sarno 1 , Simona Musella 1 , Tania Ciaglia 1 , Pietro Campiglia 1, 3 , Giuseppe Bifulco 1 , Isabel M. Gomez-Monterrey 2
Affiliation
|
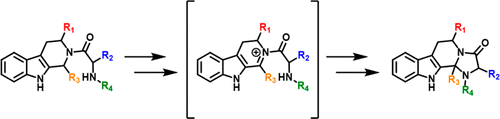
An acid- and oxidant-promoted intramolecular cyclization of a tetrahydro-β-carboline-based dipeptide has been developed to prepare new indole-fused aminoacetals. This approach involves N-acyliminium formation from readily available precursors and cyclization under mild reaction conditions. The diastereoselectivity in the formation of the products is influenced by the specific substituents of the starting reagents, which has been rationalized analyzing the energy profile of the related reactions and the relative stability of the proposed structures based on DFT computational methods.
中文翻译:

四氢-β-咔啉基二肽化合物的环稠环缩醛
已经开发了基于酸和氧化剂的基于四氢-β-咔啉的二肽分子内环化反应,以制备新的吲哚融合的氨基缩醛。该方法涉及从容易获得的前体形成N-酰基亚胺和在温和的反应条件下环化。产物形成中的非对映选择性受起始试剂的特定取代基影响,已基于DFT计算方法合理地分析了相关反应的能量分布和所提出结构的相对稳定性。
更新日期:2017-11-16
中文翻译:

四氢-β-咔啉基二肽化合物的环稠环缩醛
已经开发了基于酸和氧化剂的基于四氢-β-咔啉的二肽分子内环化反应,以制备新的吲哚融合的氨基缩醛。该方法涉及从容易获得的前体形成N-酰基亚胺和在温和的反应条件下环化。产物形成中的非对映选择性受起始试剂的特定取代基影响,已基于DFT计算方法合理地分析了相关反应的能量分布和所提出结构的相对稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号