当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Net Cycloaddition for Access to Diverse Substituted 1,5-Benzothiazepines
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acs.joc.7b02451 Yukihiro Fukata 1 , Koichi Yao 1 , Ryota Miyaji 1 , Keisuke Asano 1 , Seijiro Matsubara 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/acs.joc.7b02451 Yukihiro Fukata 1 , Koichi Yao 1 , Ryota Miyaji 1 , Keisuke Asano 1 , Seijiro Matsubara 1
Affiliation
|
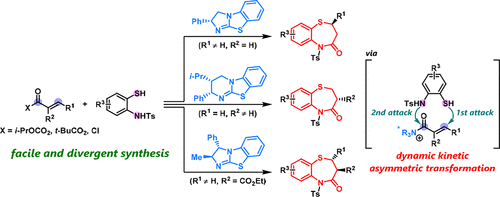
In this study, the isothiourea-catalyzed enantioselective formal [4+3] cycloaddition of various α,β-unsaturated carboxylic acid derivatives with 2-aminothiophenols was developed. Mechanistic studies suggested that the reaction proceeds via a reversible sulfa-Michael addition to α,β-unsaturated acylammonium intermediates, followed by the enantioselective formation of a seven-membered ring, enabling the facile and divergent synthesis of optically active 2- and 3-substituted 1,5-benzothiazepines. This process was demonstrated to be highly versatile, affording the corresponding products in excellent regioselectivities and high enantioselectivities. Furthermore, this method enabled the synthesis of chiral 2,3-disubstituted 1,5-benzothiazepines in high regio-, enantio-, and diastereoselectivities. Hence, this protocol can be applied for the construction of a library of useful pharmaceutical candidates.
中文翻译:

非对称净环加成反应,可制得各种取代的1,5-苯并噻氮平
在这项研究中,开发了异硫脲催化的各种α,β-不饱和羧酸衍生物与2-氨基硫酚的对映体选择性[4 + 3]环加成反应。机理研究表明,反应是通过可逆的磺胺-迈克尔加成至α,β-不饱和酰基铵中间体而进行的,随后对映选择性地形成一个七元环,从而可以轻松,发散地合成旋光的2和3取代的旋光化合物。 1,5-苯并硫氮杂s类。事实证明,该方法具有高度的通用性,可提供具有出色的区域选择性和高对映选择性的相应产品。此外,该方法使得能够以高区域,对映体和非对映体选择性合成手性2,3-二取代的1,5-苯并硫氮杂s。因此,
更新日期:2017-11-16
中文翻译:

非对称净环加成反应,可制得各种取代的1,5-苯并噻氮平
在这项研究中,开发了异硫脲催化的各种α,β-不饱和羧酸衍生物与2-氨基硫酚的对映体选择性[4 + 3]环加成反应。机理研究表明,反应是通过可逆的磺胺-迈克尔加成至α,β-不饱和酰基铵中间体而进行的,随后对映选择性地形成一个七元环,从而可以轻松,发散地合成旋光的2和3取代的旋光化合物。 1,5-苯并硫氮杂s类。事实证明,该方法具有高度的通用性,可提供具有出色的区域选择性和高对映选择性的相应产品。此外,该方法使得能够以高区域,对映体和非对映体选择性合成手性2,3-二取代的1,5-苯并硫氮杂s。因此,











































 京公网安备 11010802027423号
京公网安备 11010802027423号